Abstract
Introduction: Subjective cognitive decline (SCD) in older adults are an early risk indicator for Alzheimer’s disease or other forms of dementia, making older adults with SCD a target population for proactive interventions. The aim of this study was to determine if perceptual-cognitive training (PCT) can serve as a proactive intervention and enhance cognition in older adults with SCD.
Method: Forty-seven subjects aged 60–90 years of age were assigned to control and treatment groups using a randomised controlled trial. All the participants were asked to complete three neuropsychological assessments over a three-month period. The first assessment was prior to the PCT (T1). The second assessment (T2) was performed immediately after either seven weeks of PCT (treatment group), or after seven weeks of no training (control group). Four weeks after the completion of the PCT, a third assessment (T3) was performed to determine the veracity and persistence of any PCT benefits on cognitive performance.
Results: The results indicate a significant difference between groups at T1 and T2, wherein the treatment group has improved scores in memory tasks (e.g., CVLT-II: Immediate Free Recall; Short-Term Memory Recall, and Long-Term Memory Recall), working memory task (e.g., Digit Span Backward) and cognitive flexibility task (e.g., D-KEFS Verbal Fluency Category Switching and D-KEFS Verbal Fluency Letter Fluency). Within the treatment group the PCT scores of the last session were also significantly correlated with processing speed and cognitive flexibility. Furthermore, higher scores in memory performance were related to faster processing speeds.
Conclusion: These data suggest that PCT may serve as a proactive intervention to enhance memory, working memory and cognitive flexibility in older adults with SCD.
Keywords
Subjective cognitive decline, Perceptual-cognitive training, NeuroTracker, Memory, Processing speed, Cognitive flexibility, Working memory
Introduction
North America has a growing aging population that will introduce unique challenges for the health care system in the coming century [1]. In Canada, for example, 22.3% of the population is currently over 60 years old, and this is estimated to increase to 32.5% by 2050 [2]. While a life expectancy beyond 60 years of age has increased by about 25 years, only the first 18 years of this period are likely to be spent in good health, including good cognitive functioning [2, 3]. Generally, it is difficult to separate normal cognitive aging from pathological cognitive decline. For many people cognitive decline is associated with relatively minor and sporadic cognitive difficulties (e.g. processing speed, attention, working memory, cognitive flexibility, and episodic memory), considered normal within the spectrum of typical cognitive aging [4–6]. For some, cognitive changes are serious enough to be noticed by other people and confirmed by neuropsychological tests while these changes still do not interfere with daily life or independent function (i.e., Mild Cognitive Impairment). For others [7], cognitive decline is associated with severe cognitive deficits that impede the ability to live independently (i.e., Dementia).
Subjective cognitive decline (SCD) is a common complaint of the elderly population and may also be the earliest manifestation of Alzheimer or other forms of dementia [8]. Considerable evidence, from both behavioral and neurobiological sources, suggests that the basic cognitive domains most affected by age are executive function and memory [9, 10]. Although many older adults complain of increased memory lapses as they age not all kinds of memory are affected by normal ageing [10]. The most susceptible to brain damage and the most affected by normal aging is episodic memory [11,12]. For example, older adults tend to show more deficits on tests of free recall, to a somewhat lesser degree of difficulty in cued recall, and minimal difficulty in recognition memory. Furthermore, older adults often out-perform on attentional tasks that require flexible control, dividing or switching of attention among multiple inputs or tasks [13]. Indeed, older adults face greater difficulties in performing higher-level cognitive tasks that involve manipulation, reorganization, or integration of the contents of working memory. It seems likely that attentional resources [14], processing speed [6, 15] and the ability to inhibit irrelevant information [16] are all important functions for effective performance of these higher-level cognitive tasks.
There are many evidences that non pharmacological treatments, such as neurocognitive rehabilitation (e.g. brain stimulation techniques, computerized neurocognitive training tools), may be more effective than traditional cognitive stimulation in reducing or delaying cognitive decline in older adults [17–20]. A systematic review by Kueider and colleagues [18] assessed the efficacy of various computerized cognitive training tools, in comparison to traditional paper-and-pencil cognitive training approaches in older adults. The main benefits of the technological based training interventions were improvements in memory [21, 22], processing speed [23–27] and attention [28, 29]. Indeed, computerized cognitive training was found to be as effective as the traditional cognitive training but less labour-intensive alternative. Furthermore, computerized cognitive training had increased compliance in older adults, possibly because it is easy to access, can be used directly from home, is non-invasive, relatively inexpensive and does not require particular technological skills [18]. Therefore, introducing preventive treatments such as cognitive training programs, may have several significant benefits for an aging population [30].
Perceptual-Cognitive Training, also called Neurotracker, is a technology that was designed to enhance elite athlete performance by training their ability to track and focus on multiple moving objects in the three-dimensional visual field. This form of neurocognitive training engages visual scanning, sustained attention, divided attention, processing speed, working memory, inhibition ability, and cognitive flexibility [31–34]. Memory decline in older adults has been linked to deficits in executive processes (e.g. attention, inhibitory function, cognitive flexibility, working memory) due to their involvement in higher-level cognitive tasks [6, 9, 35]. PCT has been shown to improve different cognitive abilities in both healthy and pathological populations of young and old adults [34, 36, 37]. It was postulated that PCT may reduce or reverse the age-related cognitive decline and the aim of this study is to verify if PCT can enhance cognition in older adults with SCD.
Methods and Materials
Participants
A sample of 73 participants, between 60 and 90 years of age, was recruited using word of mouth referral and flyer distribution in the Capital Regional District (CRD) encompassing the southern tip of Vancouver Island. Print and web-based advertising were also used through the Institute on Aging and Lifelong Health at the University of Victoria. Participants were recruited from 30th of June 2017 to 13th of March 2018. The first follow-up was done on 18th of August 2017 and continued until 10th of May 2018. Socio-demographic information was collected from all participants at the baseline session (e.g., age, gender, level of education, and medical history) by completing an intake form approved by ethics committee of University of Victoria. All participants were screened for any medical, neurological, or psychiatric conditions known to affect cognitive performance in the first interview. The Mini Mental State Examination [38] was used as a screening tool (cut-off ≥ 26) to minimize the risk of including persons with preclinical dementia but as well to quantify the subjective cognitive complaints. Two tests, Activities of Daily Living [39] and Instrumental Activities of Daily Living [40], were administered to exclude subjects with possible dementia and to ensure that they were able to attend the testing and the training sessions at the University of Victoria. All participants were screened for SMCs using the Memory Complaint Questionnaire [41] and only the participants with a score of 25 or above were included in this study. All participants were screened for depression using the Geriatric Depression Scale [42] with cut-off ≥ 10, and for anxiety using the Geriatric Anxiety Inventory [43] with cut-off > 9. On self-report of a diagnosis, seven participants did not meet the inclusion criteria (i.e. one had ADHD, four subjects had Macular Degeneration; one had Anxiety Disorder, one had PTSD) and were not included in this research study. Following participant screening, only 66 subjects (female n = 48, 72.2%), aged 60 years and over (MeanAge = 73.32, SDAge = 7.58) satisfied the inclusion criteria and were enrolled in the study (e.g. over a three-month period). Eighteen subjects declined their participation to the study due to a long commitment time required. One female participant dropped out during the study due to a neurological event (e.g. a concussion outside the testing environment) and her data was removed from the analysis. The remaining 47 subjects (see Figure 1) were randomly assigned to either the treatment or control group and all subjects completed the follow-up. The method used to generate the allocation sequence was self-selection (i.e. we generated a random assignment based on the participant’s availability to commit to the study). The treatment group consisted of 25 participants between the ages of 61 and 89 years of age (female = 16; male = 9) whereas the control group consisted of 22 older adults, ages 60 to 90 years (female = 15; male = 7).
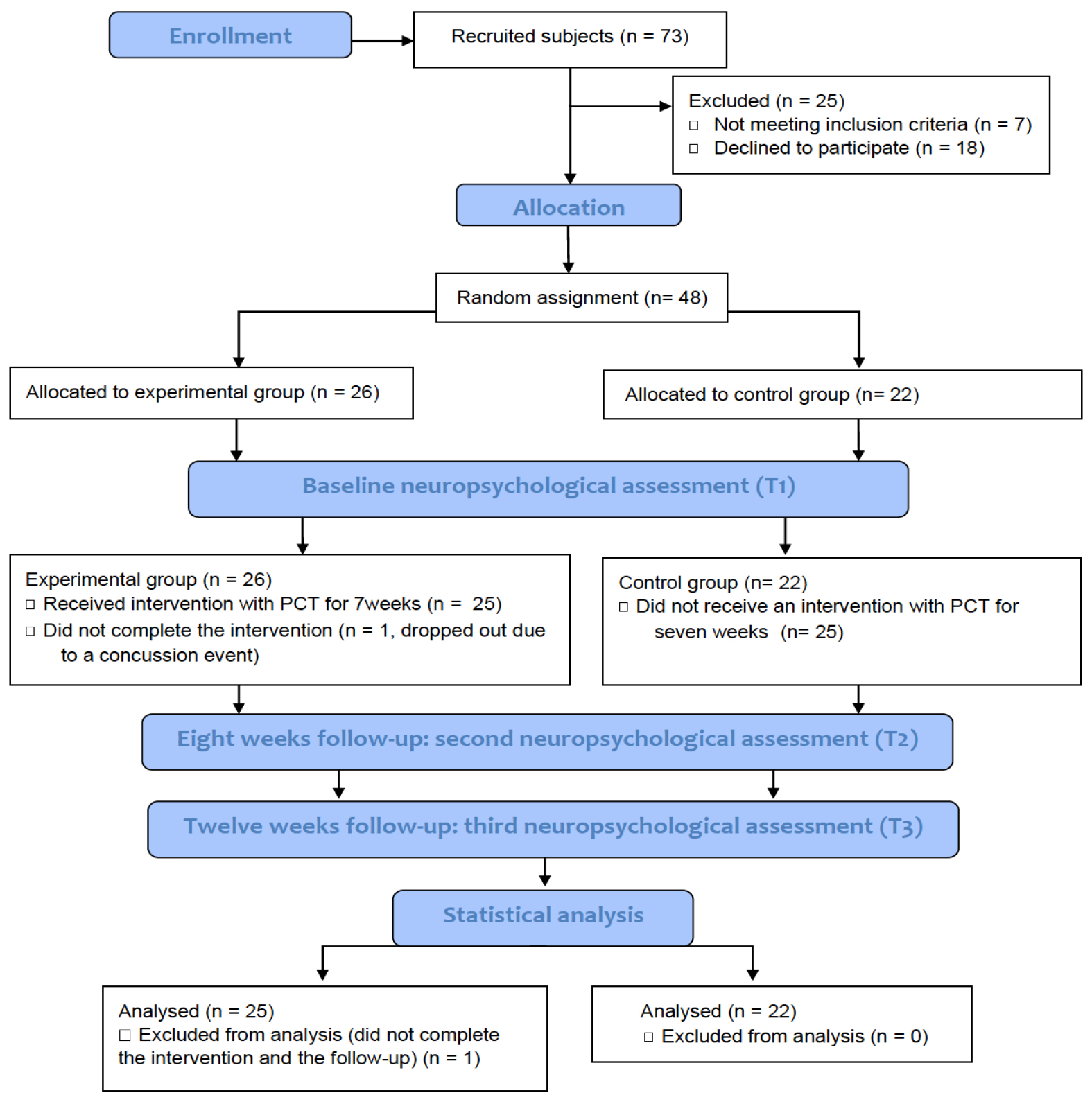
Figure 1. Flow diagram summarizing patient recruitment and progress in the study. Seventy-three individuals were initially assessed to take part in the study, of these, 18 declined to participate and 7 were excluded for not meeting inclusion criteria. The remaining 48 subjects were randomly assigned to treatment and control groups. Both groups received identical assessments, however only the treatment group received perceptual-cognitive training. Only one individual from the treatment group did not complete the training and subsequent follow-up assessments.
Procedure
This clinical study, using a parallel design, was approved on 27th June 2017 by the University of Victoria Human Research Ethics Board. The authors confirm that all ongoing and related trials for this intervention are registered (NCT03763344). This study was not registered before the enrolment of participants since UVic Research Ethics Board did not consider this study as a clinical trial but as a research study on sub-clinical population (e.g. Subjective cognitive decline).
All participants provided their informed written consent prior to participating in this study. Participants from both the treatment and control groups received a total of three neuropsychological assessments over a three-month period (see Figure 1). All the data were collected in the Concussion Laboratory of the Division of Medical Sciences, at the University of Victoria. All the tests were administered by a Doctoral Student in Clinical Neuropsychology. Considering that an essential methodological component of the training studies [44] is the use of standardized neuropsychological tests, validated and reliable measures were used. The primary outcome measure was California Verbal Learning Test, Second Edition (i.e., standard and alternate forms) [45–47]. The secondary outcome measures were Digit Span, D-KEFS Trail Making Test, D-KEFS Verbal Fluency Test (both standard and alternate forms) [45–47], and Stroop Test. Each assessment was 50–60 minutes in duration and was administered by a Doctoral Student in Clinical Neuropsychology. The first assessment was administered at baseline (T1). Then, each subject of the treatment group underwent seven weeks of perceptual cognitive training, while the control group completed seven weeks without formal training. The intervention consisted of 14 sessions of PCT each lasting 25–30 min, twice per week for seven weeks. After the seven-week time period, a second neuropsychological assessment was performed on both groups (T2). After eleven weeks, a follow-up assessment was conducted to verify whether the benefits of cognitive training endure over time (T3). We offered the PCT to both groups but at different time points (e.g. the control group engaged in the training after the follow-up assessment).
Neuropsychological Tests
Episodic memory
California Verbal Learning Test Second Edition (CVLT-II; D. C. Delis, Kramer, Kaplan, & Ober, 2000) [48] CVLT-II is a multiple-trial list-learning task that measures individuals’ episodic memory and auditory learning ability. CVLT is considered a sensitive tool in identifying subtle episodic memory difficulties. This test assesses recall and recognition of two-word lists over immediate and delayed memory trials. Standard and alternate forms of these lists exist, each with different lists of words to avoid practice effects. Each form contains two lists: list A and list B. List A is composed of 16 words divided in four different semantic categories (e.g., furniture, vegetables, methods of transportation, and animals); whereas words from the same semantic category are never presented consecutively. There are five trials using List A, and each trial requires the participant to immediately recall as many words from the list as possible. List B is a 16-word interference list, which includes different categories. List B is presented once, following the five trials of immediate recall of List A. Immediately after presentation of List B, short-delay free recall of List A is administered. Between the short-delay recall and long-delay recall, there is a 20-minute delay, which is filled with non-verbal testing (e.g., D-KEFS TMT; Stroop Test). After the non-verbal testing, long-delay free recall of List A and a recognition task (yes/no format) are administered. This list included words from both List A and List B, as well as other distractor words, where the examinee is required to identify only the words belonging to List A.
Executive Function
Delis–Kaplan Executive Function System Trail Making Test (D-KEFS TMT) (Delis, Kaplan, & Kramer, 2001) [49] is a pencil and paper task, used to evaluate aspects of cognition including processing speed, motor speed and cognitive flexibility. It involves a series of five conditions: visual scanning, number sequencing, letter sequencing, number-letter switching, and motor speed. In the visual scanning condition, examinees must cross out all the threes that appear on the response sheet mixed with other numbers. In the number sequencing condition, examinees draw a line connecting the numbers 1–16 in counting order while avoiding distractor letters that appear on the same page. The letter sequencing condition requires examinees to connect the letters A through P, with distractor numbers presented on the page. In the number-letter switching condition, examinees switch back and forth between connecting numbers in counting order and letters in alphabetical order (i.e., 1, A, 2, B, etc., to 16, P). This condition requires the ability to switch mentally between numerical and alphabetical sequences and provides an assessment of the participant’s cognitive flexibility. Finally, the examinee completes a motor speed condition in which he/she has to trace over a dotted line connecting circles on the page as quickly as possible. This final section assesses their graphomotor speed.
Delis–Kaplan Executive Function System Verbal Fluency Test (D-KEFS VFT) (Delis, Kaplan, & Kramer, 2001) [49] is a short test of verbal functioning that measures processing speed and cognitive flexibility. There are three conditions: Letter Fluency, Category Fluency, and Category Switching. In all three conditions, the examinees are given 60 seconds to generate as many words following a semantic cue (e.g., specific category), a phonemic cue (e.g. starting with a certain letter) or alternating between two categories a task, which requires a certain amount of mental flexibility.
Digit Span Test is a measure of working memory consisting of 16 trials; eight in Digit Span Forward, and eight in Digit Span Backward. In both conditions, the examiner reads out a series of numbers, ranging from 2–9 digits in sequence. In the forward condition, the participant is asked to repeat the numbers verbatim as stated by the examiner at the end of each trial. In the backward condition, the participant is asked to repeat the numbers in the reverse order stated by the examiner.
Stroop test is used to measure selective attention, psychomotor speed and cognitive flexibility [50]. In this study, the Stroop test was delivered using the Encephal App [51], which adheres to the same principles as the classic Stroop version [52]. Subjects are required to identify the ink colour of discordant-colour words (red, blue, or green). The task consists of two parts: the Stroop effect turned off (i.e. the examinees name the colour of the ink of a set of number signs) and the Stroop effect turned on (i.e. series of colour words “Red”, “Blue”, “Green” are presented in an incongruent coloured ink). In this task, the examinee must inhibit the automatic tendency of reading in order to name correctly the colour of the ink. The placement of the words and number signs are randomized and change position on the screen with each new stimulus. The order of the responses on the bottom of the screen that examinees need to respond to are randomized and shifts in order with each new stimulus. The examinees are not instructed that the order of the response options shift with each new screen, requiring more focus and mental flexibility to the changing stimuli.
Perceptual-Cognitive Training
NeuroTracker is a computerized perceptual-cognitive training system developed by Jocelyn Faubert of University of Montreal [33, 53, 54]. This training is based on a computerized 3D Multiple Object Tracking (3D-MOT) model that follows two principles: isolation and overloading. Isolation training uses limited and consistent cognitive load, while overloading challenges the subject by training them at levels beyond their current ability in order to increase cognitive functioning. Previous studies have indicated that the training effect is reduced if isolation and overloading are not applied to the task [55, 56].
Each PCT session consists of three series of 20 trials in which the subject wears 3D glasses and tracks four spheres among four identical distractors that move in a 3D volumetric cube on the screen. In the first phase, all eight spheres are stationary on the screen, then the four targets briefly change to red and after two seconds revert to yellow. The four target spheres must be tracked as they moved in a linear trajectory for eight seconds. After this, the spheres stop moving and the subject is asked to identify the four targets.
The sessions are based on a staircase procedure [57], in which an algorithm shifts the speed of the target spheres in regard to the participants’ performance (i.e., overloading principle). If all targets were correctly identified, the speed of the movement of the spheres increases by 0.05log, whereas with each incorrect response the speed decreases by 0.05log.
Data Analysis
IBM SPSS Statistics v22.0 and R Software were used for the statistical analyses. Descriptive statistics were computed and full statistical diagnostics carried out to check for adequate distributions, out-of-range values, missing values and outlier checks well as overall standard deviations and standard errors values. Such diagnostics were iteratively conducted on the data collected upon completion of the three assessments: prior the intervention (T1), after the seven weeks of training (T2), and four weeks post-intervention (T3). In particular, box plots for each group and dependent measures were used to identify critical outliers pre-, post-training, and after a month of follow-up. It was decided to constrain outliers values with more than 3 standard deviations above or below the mean. The Trimming method [58, 59] was used to replace the outliers found by the second-highest value from the respective cognitive task group (e.g. CVLT-II, D-KEFS VFT) or by the second-lowest value from the tasks measured in seconds (e.g. D-KEFS TMT, Stroop Test). Data of a subject that dropped out in the middle of the intervention for a concussion reason was removed. Following up the statistical diagnostics and data screening, a first series of independent t-tests were performed on the data at T1 to verify that both groups were equal at baseline in terms of age, education, global cognitive efficiency (MMSE), memory complaints, and leisure activities prior to the intervention. Next, a factorial between-within subject differences (i.e. treatment and control differences across time T1, T2 and T3) were examined by a Doubly Factorial MANOVA. Finally, univariate Within-Subjects Contrasts further examined cognitive abilities that displayed a linear trend in the treatment group (p < .05).
Research expectations were 1) to support a construction of a balanced design with no multivariate or univariate F test differences at baseline (T1) between the two groups); 2) to detect significant multivariate and univariate effects at T1 and T2 between groups (expectation is that treatment group would perform better); 3) to identify some linear trends for the experimental group across T2 and T3. Notably, testing for significant multivariate results at T2 and T3 (if any) might also provide some indication on the potential future use of a linear composite of such DVs to study differences across patients instead of relying on single univariate measures. A one way repeated measures (RM) ANOVA (Time: Session 1 to Session 14) to analyse the PCT performance for the treatment group. Additionally, a series of stepwise linear regressions were used to verify if PCT training scores predicted cognitive performance for the treatment group. Where appropriate, the assumption of sphericity was tested and where violations occurred a Greenhouse-Geisser correction was applied.
Results
Descriptive statistics
The analyses were performed at the group level on all 47 subjects that concluded the study. The data of the participant that dropped-out was removed from the analysis. An independent t-test was performed between the control and the treatment groups and showed no differences (all p > .05) for age, global cognitive efficiency, and memory complaints prior to the intervention (see Table 1).
Table 1. Demographic information for control (n = 22) and treatment (n = 25) groups.
|
|
Control group (n = 22) |
Treatment group (n = 25) |
U test / t-test |
|||||
|
Variables |
M (SD) |
95% CI |
M (SD) |
95% CI |
t |
p |
||
|
Age |
72.14 (6.23) |
69.37 |
74.9 |
74.36 (8.73) |
70.75 |
77.96 |
1.01 |
.137 |
|
Education |
15.73 (2.81) |
14.47 |
16.97 |
16.40 (4.03) |
14.73 |
18.06 |
.65 |
.516 |
|
MMSE |
29.27 (.70) |
28.96 |
29.58 |
29.24 (1.30) |
28.7 |
29.77 |
-.10 |
.914 |
|
|
Mdn |
|
|
Mdn |
|
|
U test |
p |
|
MAC-Q |
26 (7) |
|
|
27 (8) |
|
|
212 |
.170 |
|
MMSE: Mini Mental State Examination; MAC-Q: Memory Complaint Questionnaire |
||||||||
Similarly, no differences (all p > .05) were found between groups at baseline for the major components that could contribute to their cognitive reserve (education and leisure activities). Further, the Multivariate difference analysis at baseline (T1) shows no differences (all p > .05) between groups in terms of cognitive functioning. Overall such results would well represent an experimental condition of favorable balanced design (Table 2).
Table 2. Multivariate difference at baseline (T1) between groups.
|
Cognitive variables |
Control group |
Treatment group |
Pairwise comparisons |
|
|
M (SD) |
M (SD) |
p |
F value |
|
|
CVLT-II List A IFR |
52.50 (2.42) |
55.28 (2.26) |
.410 |
.703 |
|
CVLT-II List A SDFR |
10.54 (.79) |
11.92 (.74) |
.210 |
1.617 |
|
CVLT-II List A LDFR |
11.32 (.63) |
11.72 (.59) |
.644 |
.216 |
|
CVLT-II List A LDR |
15.50 (.17) |
15.13 (.16) |
.131 |
2.269 |
|
CVLT-II List A LDR FPE |
2.22 (.46) |
1.25 (.43) |
.134 |
2.332 |
|
DIGIT SPAN F. |
6.50 (.23) |
6.80 (.21) |
.343 |
.920 |
|
DIGIT SPAN B. |
5.23 (.28) |
5.70 (.26) |
.244 |
1.396 |
|
TOTAL DIGIT SPAN |
11.68 (.44) |
12.48 (.40) |
.190 |
1.794 |
|
D-KEFS TMT: VS |
24.36 (1.40) |
25.96 (1.26) |
.390 |
.764 |
|
D-KEFS TMT: NS |
46.07 (3.58) |
38.80 (3.36) |
.145 |
2.197 |
|
D-KEFS TMT: LS |
42.73 (3.75) |
37.92 (3.53) |
.560 |
.871 |
|
D-KEFS TMT: NLS |
94.90 (10.82) |
98.24 (10.15) |
.823 |
.050 |
|
D-KEFS TMT: MS |
27.18 (2.32) |
31.03 (2.18) |
.232 |
1.467 |
|
D-KEFS VFT: LF |
42.64 (2.12) |
44.64 (1.99) |
.495 |
.473 |
|
D-KEFS VFT: CF |
37.64 (1.83) |
38.90 (1.71) |
.625 |
.242 |
|
D-KEFS VFT: CS |
11.72 (.74) |
11.36 (.70) |
.720 |
.132 |
|
STROOP TEST OFF |
83.35 (3.43) |
85.75 (3.21) |
.612 |
.261 |
|
STROOP TEST ON |
100.12 (4.30) |
103.63 (4.03) |
.560 |
.354 |
|
*CVLT-II List A IFR – Immediate Free Recall Trials 1–5; CVLT-II List A SDFR – Short-Delay Free Recall; CVLT-II List A LDFR – Long-Delay Free Recall; CVLT-II List A LDR – Long-Delay Yes/No Recognition; CVLT-II List A LDR FPE – Long-Delay Recognition False Positive Errors; DIGIT SPAN F. – Digit Span Forward; DIGIT SPAN B. – Digit Span Backward; D-KEFS TMT:VS – Visual Scanning; D-KEFS TMT: NS-Number Sequencing; D-KEFS TMT: LS – Letter Sequencing; D-KEFS TMT: NLS – Number-Letter Switching; D-KEFS TMT: MS – Motor Speed; D-KEFS VFT: LF – Letter Fluency; D-KEFS VFT: CF – Category Fluency; D-KEFS VFT: CS – Category Switching. |
||||
Factorial Multivariate Analysis
A Factorial Doubly MANOVA was conducted (i.e. 2×3 groups: control, experimental; time: T1, T2 and T3) to examine the transferability of PCT benefits on cognitive performance. Using Wilk’s lambda, there was a significant multivariate effect of interaction between groups and time for the cognitive variables considered in this study Λ =.401, F = (38, 144) = 2.20, p= .000, ɳp2 = 1. To further explore this significant MANOVA interaction a set of separate follow-up univariate ANOVAs (simple main effects analysis) on the cognitive variables revealed significant treatment effects between groups on CVLT-II Immediate Free Recall Trials 1–5; CVLT-II Short-Delay Free Recall; CVLT-II Long-Delay Free Recall; CVLT-II Recognition; D-KEFS VFT Letter Fluency, D-KEFS VFT Category Switching, D-KEFS TMT Visual Scan, D-KEFS TMT (Table 3). Notably, due to the exploratory nature of such analysis all such individual F-value tests have to be further investigated to confirm the various target variable contributions to the MANOVA model findings so far.
Table 3. Univariate test between groups in time.
|
|
Sum of Squares |
df |
Mean Square |
F |
p |
Partial Eta Squared |
Observed Power |
|
CVLT-II List A/B IFR |
290.16 |
2 |
145.080 |
3.247 |
.043* |
.067 |
.605 |
|
CVLT-II List A/B SDFR |
36.008 |
2 |
18.004 |
5.016 |
.009** |
.100 |
.803 |
|
CVLT-II List A/B LDFR |
44.905 |
2 |
22.452 |
6.433 |
.002** |
.125 |
.895 |
|
CVLT-II List A/B LDR |
4.715 |
2 |
2.357 |
4.474 |
.014* |
.090 |
.753 |
|
CVLT-II List A/B LDR FPE |
7.668 |
2 |
3.844 |
.829 |
.440 |
.018 |
1.658 |
|
DIGIT SPAN F. |
.172 |
2 |
.086 |
.165 |
.848 |
.004 |
.075 |
|
DIGIT SPAN B. |
3.190 |
2 |
1.595 |
1.716 |
.186 |
.037 |
.352 |
|
TOTAL DIGIT SPAN |
3.341 |
2 |
1.671 |
1.256 |
.290 |
.027 |
.267 |
|
D-KEFS VFT: LF |
245.452 |
2 |
122.726 |
3.752 |
.027* |
.077 |
.672 |
|
D-KEFS VFT: CF |
18.428 |
2 |
9.124 |
.397 |
.673 |
.009 |
.112 |
|
D-KEFS VFT: CS |
48.512 |
2 |
24.256 |
3.551 |
.033* |
.073 |
.647 |
|
D-KEFS TMT:VS |
103.179 |
2 |
51.590 |
3.753 |
.027* |
.077 |
.672 |
|
D-KEFS TMT:NS |
532.787 |
2 |
266.394 |
2.210 |
.116 |
.047 |
.440 |
|
D-KEFS TMT:LS |
203.779 |
|
101.890 |
1.056 |
.352 |
.023 |
.230 |
|
D-KEFS TMT: NLS |
2.953.761 |
2 |
1.476.880 |
2.457 |
.091 |
.052 |
.483 |
|
D-KEFS TMT: MS |
260.530 |
2 |
130.265 |
2.740 |
.070 |
.057 |
.529 |
|
STROOP TEST OFF |
242.613 |
2 |
121.306 |
1.016 |
.366 |
.022 |
.222 |
|
STROOP TEST ON |
202206 |
2 |
101103 |
.658 |
.520 |
.014 |
.157 |
|
*indicates significance at the 0.05 level **indicates significance at the 0.01 level CVLT-II List A IFR – Immediate Free Recall Trials 1–5; CVLT-II List A SDFR – Short-Delay Free Recall; CVLT-II List A LDFR – Long-Delay Free Recall; CVLT-II List A LDR – Long-Delay Yes/No Recognition; CVLT-II List A LDR FPE – Long-Delay Recognition False Positive Errors; DIGIT SPAN F. – Digit Span Forward; DIGIT SPAN B. – Digit Span Backward; D-KEFS TMT:VS – Visual Scanning; D-KEFS TMT: NS-Number Sequencing; D-KEFS TMT: LS – Letter Sequencing; D-KEFS TMT: NLS – Number-Letter Switching; D-KEFS TMT: MS – Motor Speed; D-KEFS VFT: LF – Letter Fluency; D-KEFS VFT: CF – Category Fluency; D-KEFS VFT: CS – Category Switching. |
|||||||
Treatment-Control Groups differences
To dissect further the univariate F tests main effects analyses discussed above, a series ofsimple contrasts comparisons across the treatment and control groups were carried out separately at T2 and T3 respectively. At T2 a evaluations significant difference was observed in the scores of CVLT-II long delay recognition memory task between control (M=15.15; SE=.15) and treatment (M=15.79; SE=.14) groups F(25)=7.190, p=.010 at T2. The observed power of this significant difference represents a large-sized effect (Table 4). A significant difference was also noticed in verbal cognitive flexibility performance, such as D-KEFS verbal fluency category switching task, between the control (M=10.83; SE=.66) and treatment (M=12.64; SE=.62) groups F(25)=4.065, p=.050 at T2. The observed power of this significant difference represents a medium-sized effect (Table 4). A significant difference was observed in sustained attention task, such as STROOP TEST OFF, between the control (M=78.75; SE=3.20) and treatment (M=87.53; SE=3.01) groups F(25)=4.065, p=.050 at T2. The observed power of this significant difference represents a medium-sized effect (Table 4). Furthermore, it seems to be a trend of higher performance for the treatment group compared to the control group in retrieving words in a memory task such as CVLT-II Immediate Free Recall (e.g. CVLT-II List A/B IFR). Although this difference represents a medium-sized effect, it does not reach statistical significance (p < .05).
Table 4. Pairwise comparisons between groups T2.
|
Cognitive variables |
Control group |
Treatment group |
Pairwise comparison |
|||
|
M (SE) |
M (SE) |
p |
F |
Partial Eta Squared |
Observed Power |
|
|
CVLT-II List A/B IFR |
52.73 (2.15) |
58.04 (2.01) |
.078 |
3.254 |
.67 |
.423 |
|
CVLT-II List A/B SDFR |
10.50 (.67) |
11.84 (.63) |
.154 |
2.097 |
.045 |
.294 |
|
CVLT-II List A/B LDFR |
10.97 (.71) |
12.36 (.67) |
.158 |
2.062 |
.044 |
.290 |
|
CVLT-II List A/B LDR |
15.15 (.15) |
15.79 (.14) |
.010* |
7.190 |
.138 |
.747 |
|
CVLT-II List A/B LDR FPE |
3.73 (.92) |
1.70 (.87) |
.113 |
2.616 |
.113 |
.353 |
|
DIGIT SPAN F. |
6.59 (.23) |
6.72 (.22) |
.690 |
.161 |
.004 |
.068 |
|
DIGIT SPAN B. |
5.09 (.31) |
5.16 (.29) |
.870 |
.027 |
.001 |
.053 |
|
TOTAL DIGIT SPAN |
11.64 (.45) |
11.90 (.42) |
.693 |
.158 |
.004 |
.068 |
|
D-KEFS VFT: LF |
41.00 (2.31) |
44.80 (2.16) |
.236 |
1.445 |
.031 |
.218 |
|
D-KEFS VFT: CF |
40.46 (1.82) |
39.92 (1.71) |
.831 |
.046 |
.001 |
.055 |
|
D-KEFS VFT: CS |
10.83 (.66) |
12.64 (.62) |
.050* |
4.065 |
.083 |
.505 |
|
D-KEFS TMT: VS |
23.49 (1.22) |
23.20 (1.15) |
.865 |
.029 |
.001 |
.053 |
|
D-KEFS TMT: NS |
34.23 (2.35) |
36.40 (2.21) |
.503 |
.455 |
.010 |
.101 |
|
D-KEFS TMT: LS |
37.99 (3.63) |
37.23 (3.41) |
.880 |
.023 |
.001 |
.053 |
|
D-KEFS TMT: NLS |
93.64 (7.92) |
86.77 (7.43) |
.530 |
.400 |
.009 |
.095 |
|
D-KEFS TMT: MS |
27.31 (1.85) |
25.17 (1.74) |
.403 |
.713 |
.016 |
.131 |
|
STROOP TEST OFF |
78.75 (3.20) |
87.53 (3.01) |
.050* |
4.002 |
.082 |
.499 |
|
STROOP TEST ON |
96.08 (4.50) |
104.7 (4.22) |
.169 |
1.952 |
.042 |
.277 |
|
*indicates significance at the 0.05 level VLT-II List A IFR – Immediate Free Recall Trials 1–5; CVLT-II List A SDFR – Short-Delay Free Recall; CVLT-II List A LDFR – Long-Delay Free Recall; CVLT-II List A LDR – Long-Delay Yes/No Recognition; CVLT-II List A LDR FPE – Long-Delay Recognition False Positive Errors; DIGIT SPAN F. – Digit Span Forward; DIGIT SPAN B. – Digit Span Backward; D-KEFS TMT:VS – Visual Scanning; D-KEFS TMT: NS-Number Sequencing; D-KEFS TMT: LS – Letter Sequencing; D-KEFS TMT: NLS – Number-Letter Switching; D-KEFS TMT: MS – Motor Speed; D-KEFS VFT: LF – Letter Fluency; D-KEFS VFT: CF – Category Fluency; D-KEFS VFT: CS – Category Switching. |
||||||
At T3 significant differences between groups were observed in the scores of CVLT-II immediate free recall memory task F(25)=8.545, p=.005, CVLT-II short delay free recall F(25)=15.690, p=.000, and CVLT-II long delay free recall task F(25)=13.007, p=.001. The number of words recalled by the treatment group is higher compared to controls and the observed power of these significant differences represents a large- sized effect (Table 5). A significant difference between groups at T3 was also noticed in the scores of working memory task (i.e. Digit Span Backward) F(25)=5.700, p = .112. The number of digits repeated by the participants of the treatment group is higher compared to controls and the observed power of this significant difference represents a large-sized effect (Table 5). Similarly, a significant difference between groups at T3 was also noticed in a verbal task that requires a certain amount of cognitive flexibility (i.e. D-KEFS verbal fluency category switching task) F(25)=7.032, p=.011. In this task the participants of the treatment group generate a higher number of words compared to controls and the observed power of this significant difference represents a large- sized effect (Table 5).
Table 5. Pairwise comparisons between groups T3.
|
Cognitive variables |
Control group |
Treatment group |
Pairwise comparison |
|||
|
M (SE) |
M (SE) |
p |
F |
Partial Eta Squared |
Observed Power |
|
|
CVLT-II List A/B IFR |
51.94 (2.43) |
61.68 (2.27) |
.005** |
8.545 |
.160 |
.816 |
|
CVLT-II List A/B SDFR |
9.46 (.65) |
12.96 (.61) |
.000** |
15.690 |
.259 |
.972 |
|
CVLT-II List A/B LDFR |
10.18 (.63) |
13.32 (.60) |
.001** |
13.007 |
.224 |
.942 |
|
CVLT-II List A/B LDR |
15.20 (.22) |
15.29 (.20) |
.566 |
.334 |
.007 |
.087 |
|
CVLT-II List A/B LDR FPE |
2.42 (.47) |
1.24 (.44) |
.075 |
3.325 |
.069 |
.430 |
|
DIGIT SPAN F. |
6.64 (.22) |
6.84 (.21) |
.505 |
.451 |
.010 |
.101 |
|
DIGIT SPAN B. |
5.27 (.25) |
6.08 (.23) |
.021* |
5.700 |
.112 |
.647 |
|
TOTAL DIGIT SPAN |
11.96 (.41) |
12.92 (.39) |
.093 |
2.943 |
.061 |
.389 |
|
D-KEFS VFT: LF |
40.91 (2.48) |
49.20 (2.32) |
.019* |
5.952 |
.117 |
.665 |
|
D-KEFS VFT: CF |
39.18 (1.60) |
39.40 (1.50) |
.921 |
.010 |
.000 |
.051 |
|
D-KEFS VFT: CS |
10.41 (.70) |
12.76 (.61) |
.011* |
7.032 |
.135 |
.737 |
|
D-KEFS TMT: VS |
25.23 (1.24) |
22.64 (1.17) |
.135 |
2.311 |
.049 |
.319 |
|
D-KEFS TMT: NS |
35.96 (2.64) |
32.32 (2.48) |
.321 |
1.009 |
.022 |
.166 |
|
D-KEFS TMT: LS |
39.51 (2.70) |
33.01 (2.53) |
.086 |
3.081 |
.064 |
.404 |
|
D-KEFS TMT: NLS |
100.22 (7.85) |
81.12 (7.37) |
.083 |
3.150 |
.065 |
.412 |
|
D-KEFS TMT: MS |
26.36 (1.70) |
24.70 (1.60) |
.475 |
.520 |
.011 |
.109 |
|
STROOP TEST OFF |
78.75 (3.20) |
83.43 (2.83) |
.246 |
1.382 |
.030 |
.210 |
|
STROOP TEST ON |
93.70 (4.17) |
97.19 (3.92) |
.541 |
.380 |
.008 |
.093 |
|
*indicates significance at the 0.05 level **indicates significance at the 0.01 level VLT-II List A IFR – Immediate Free Recall Trials 1–5; CVLT-II List A SDFR – Short-Delay Free Recall; CVLT-II List A LDFR – Long-Delay Free Recall; CVLT-II List A LDR – Long-Delay Yes/No Recognition; CVLT-II List A LDR FPE – Long-Delay Recognition False Positive Errors; DIGIT SPAN F. – Digit Span Forward; DIGIT SPAN B. – Digit Span Backward; D-KEFS TMT:VS – Visual Scanning; D-KEFS TMT: NS-Number Sequencing; D-KEFS TMT: LS – Letter Sequencing; D-KEFS TMT: NLS – Number-Letter Switching; D-KEFS TMT: MS – Motor Speed; D-KEFS VFT: LF – Letter Fluency; D-KEFS VFT: CF – Category Fluency; D-KEFS VFT: CS – Category Switching. |
||||||
Furthermore, it seems to be a trend of higher performance for the treatment group compared to the control group in tasks such as long-delay memory recognition (e.g. CVLT-II List A/B LDR FPE), working memory (e.g. Total Digit Span), visual cognitive flexibility (e.g. D-KEFS TMT: LS) and visual processing speed (e.g. D-KEFS TMT: NLS), but did not reach statistical significance (p < .05).
Descriptive Trend analysis across groups
For exploratory purposes the descriptive linear trends over the 3 time periods (T1, T2 and T3) are reported in (Figures 2, 3 and 4). The figures 2 and 3 show the upwards increase in the estimated marginal means for “CVLT Long Delay Memory Recall” (i.e. episodic memory) and “D-KEFS VF Category Switching” (i.e. cognitive flexibility) between the treatment group versus the control group. The latter one instead depicts the downward and expected linear trend of “D-KEFS TMT Number-Letter Switching” (i.e. cognitive flexibility). Such descriptive trends (Table 6) mirror various results in the dissected MANOVA pairwise comparisons across the groups and time windows. Clearly more research is needed to further understand potential clinical impact of such potential trends. Nevertheless, such trends are encouraging and require further research in the near future. Such trends, if present could be highly relevant to verify the magnitude of improvement across different time periods and adequate clinical design tailored to such processes.
Table 6. Linear trend analysis results of the cognitive performance in the treatment group.
|
Cognitive variables |
Treatment group (n = 25) |
|
|||||
|
T1 M (SD) |
T2 M (SD) |
T3 M (SD) |
F |
p |
ɳp2 |
Power |
|
|
CVLT-II List A/B IFR |
55.28 (2.26) |
58.04 (2.01) |
61.68 (2.27) |
15.23 (1, 24) |
.001** |
.388 |
.963 |
|
CVLT-II List A/B SDFR |
11.92 (.74) |
11.84 (.63) |
12.96 (.61) |
3.84 (1, 24) |
.062 |
.138 |
.469 |
|
CVLT-II List A/B LDFR |
11.72 (.59) |
12.36 (.67) |
13.32 (.60) |
17.45 (1, 24) |
.000** |
.421 |
.980 |
|
CVLT-II List A/B LDR |
15.13 (.16) |
15.79 (.14) |
15.29 (.20) |
.775 (1, 24) |
.388 |
.031 |
.135 |
|
CVLT-II List A/B LDR FPE |
1.25 (.43) |
1.70 (.87) |
1.24 (.44) |
.002 (1, 24) |
.962 |
.000 |
.050 |
|
DIGIT SPAN F. |
6.80 (.21) |
6.72 (.22) |
6.84 (.21) |
.033 (1, 24) |
.857 |
.001 |
.054 |
|
DIGIT SPAN B. |
5.70 (.26) |
5.16 (.29) |
6.08 (.23) |
2.087 (1. 24) |
.161 |
.080 |
.284 |
|
TOTAL DIGIT SPAN |
12.48 (.40) |
11.90 (.42) |
12.92 (.39) |
1.160 (1, 24) |
.292 |
.046 |
.179 |
|
D-KEFS VFT: LF |
25.96 (1.26) |
44.80 (2.16) |
49.20 (2.32) |
7.03 (1, 24) |
.014* |
.227 |
.721 |
|
D-KEFS VFT: CF |
38.80 (3.36) |
39.92 (1.71) |
39.40 (1.50) |
.306 (1, 24) |
.585 |
.013 |
.083 |
|
D-KEFS VFT: CS |
37.92 (3.53) |
12.64 (.62) |
12.76 (.61) |
3.56 (1, 24) |
.071 |
.129 |
.441 |
|
D-KEFS TMT: VS |
98.24 (10.15) |
23.20 (1.15) |
22.64 (1.17) |
8.90 (1, 24) |
.006* |
.271 |
.817 |
|
D-KEFS TMT: NS |
31.03 (2.18) |
36.40 (2.21) |
32.32 (2.48) |
3.45 (1, 24) |
.075 |
.126 |
.431 |
|
D-KEFS TMT: LS |
44.64 (1.99) |
37.23 (3.41) |
33.01 (2.53) |
3.96 (1,24) |
.058 |
.142 |
481 |
|
D-KEFS TMT: NLS |
38.90 (1.71) |
86.77 (7.43) |
81.12 (7.37) |
4.88 (1,24) |
.037* |
.129 |
.564 |
|
D-KEFS TMT: MS |
11.36 (.70) |
25.17 (1.74) |
24.70 (1.60) |
7.66 (1,24) |
.011* |
.242 |
.757 |
|
STROOP TEST OFF |
85.75 (3.21) |
87.53 (3.01) |
83.43 (2.83) |
.416 (1,24) |
.525 |
.017 |
.095 |
|
STROOP TEST ON |
103.63 (4.03) |
104.7 (4.22) |
97.19 (3.92) |
2.65 (1,24) |
.116 |
.100 |
.347 |
|
*indicates significance at the 0.05 level **indicates significance at the 0.01 level VLT-II List A IFR – Immediate Free Recall Trials 1–5; CVLT-II List A SDFR – Short-Delay Free Recall; CVLT-II List A LDFR – Long-Delay Free Recall; CVLT-II List A LDR – Long-Delay Yes/No Recognition; CVLT-II List A LDR FPE – Long-Delay Recognition False Positive Errors; DIGIT SPAN F. – Digit Span Forward; DIGIT SPAN B. – Digit Span Backward; D-KEFS TMT:VS – Visual Scanning; D-KEFS TMT: NS-Number Sequencing; D-KEFS TMT: LS – Letter Sequencing; D-KEFS TMT: NLS – Number-Letter Switching; D-KEFS TMT: MS – Motor Speed; D-KEFS VFT: LF – Letter Fluency; D-KEFS VFT: CF – Category Fluency; D-KEFS VFT: CS – Category Switching. |
|||||||
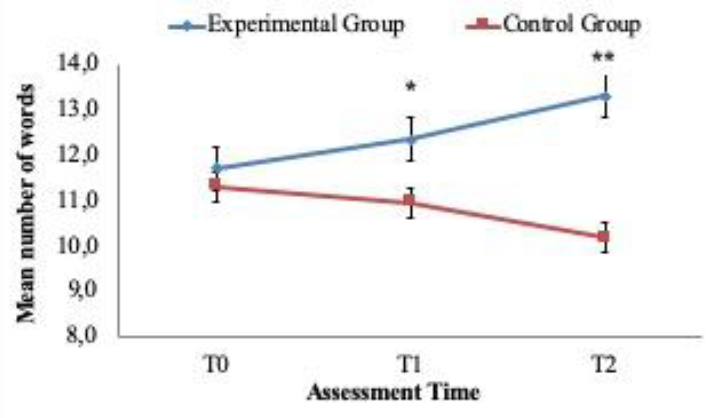
Figure 2. Linear trend analysis. Long-delay memory recall measured with CVLT-II List A/B Long-Delay.
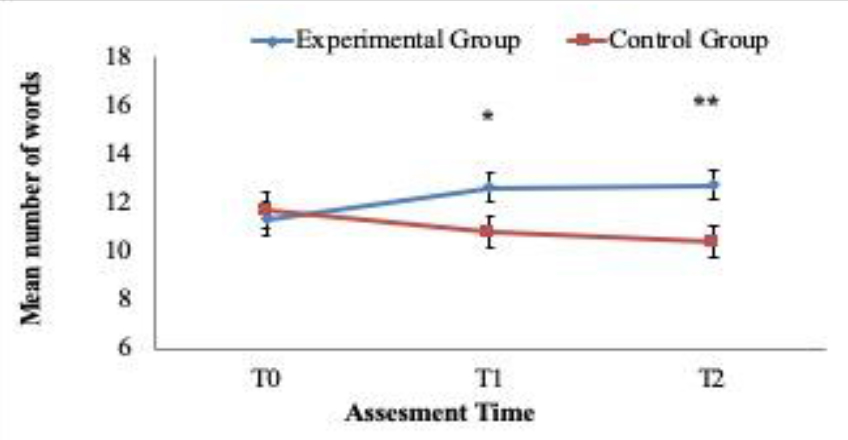
Figure 3. Linear trend analysis. Verbal cognitive flexibility measured with D-KEFS Verbal Fluency Test: Category Switching.
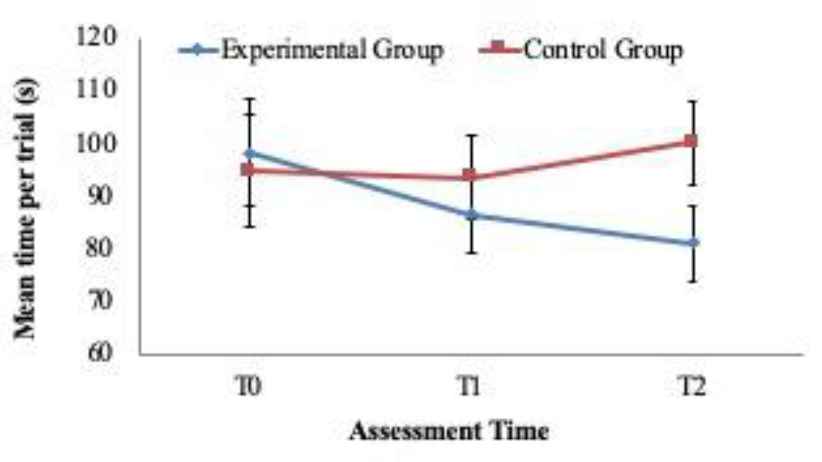
Figure 4. Linear trend analysis. Visual cognitive flexibility measured with D-KEFS Trail Making Test: Number-Letter Switching.
Perceptual-cognitive training (PCT) performance analyses
A visual inspection of the PCT data suggested that the treatment group showed improvements in performance across sessions (Figure 3). To affirm this, for example, the PCT thresholds showed an apparent logarithmic trend, characteristic of a good learning curve (R2 = .92) [37]. Further, a one-way (Time: Session 1 to Session 14) RM ANOVA was used to statistically analyse PCT performance. This analysis revealed a significant change in performance F(1, 13) = 49.95, p = .000 from Session 1 to Session 14, corroborating the significant presence of a trend across the sessions (Figure 5).
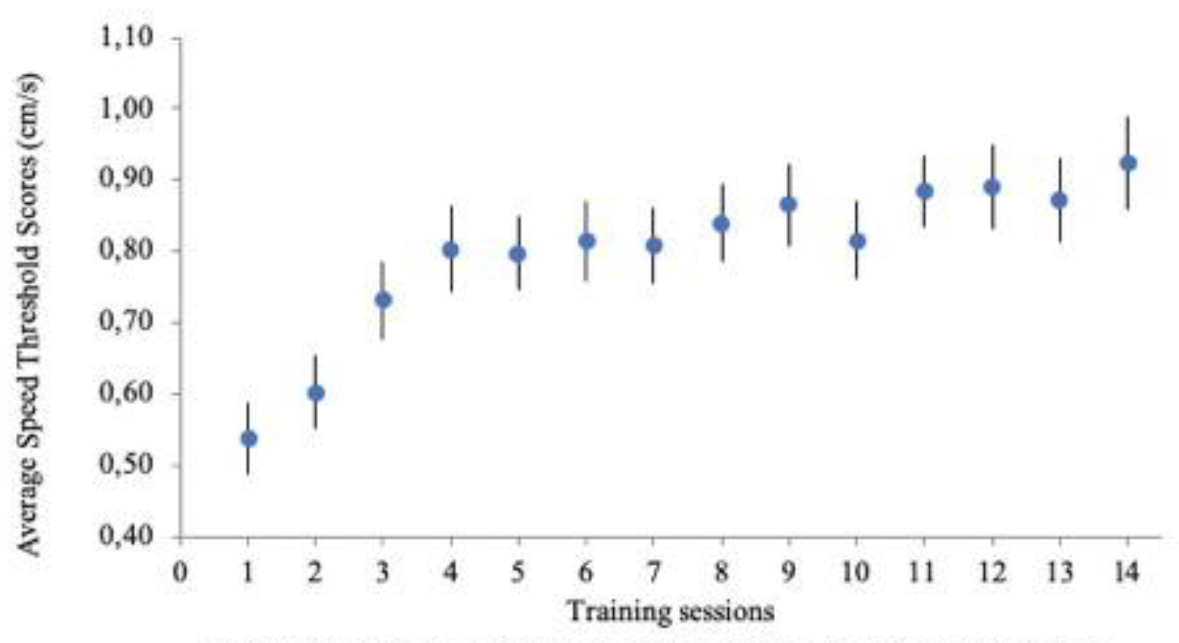
Figure 5. Average speed threshold scores with PCT from the treatment group participants (n= 25). Speed thresholds are plotted for subjects in the treatment group. Subjects received two training sessions a week over a 7 week period, for a total of 14 sessions. Note how subjects show a marked improvement in performance after session 2 that persists for the duration of the training period.
Relationship between PCT performance and enhancement in cognitive functioning in the treatment group
Finally, a series of stepwise regression were used to verify if PCT scores predicted cognitive performance for the treatment group. Results showed that PCT scores predicted increasing performance in Digit Span Backward task F(1, 23) = 17.429, p = .000b, with an R2 of .442. Further, results revealed a negative relationship between the performance in the last PCT session performance and in the D-KEFS TMT Visual Scanning (r = – .366; p = .036) and D-KEFS TMT Number Sequencing (r = – .364; p = .037). Similarly, a positive relationship was found between performance in the last PCT session and D-KEFS Letter Fluency (r = .387; p = .028) and CVLT-II Long Delay Recall (r = .391; p = .027) (Table 7).
Table 7. Bivariate correlation between the cognitive tasks in the control group.
|
Cognitive variables |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
|
1 |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
r = .570** |
1 |
|
|
|
|
|
|
|
|
|
|
|
|
3 |
r = .329 |
r = .526 |
1 |
|
|
|
|
|
|
|
|
|
|
|
4 |
r = -.620** |
r = -.578** |
r = -.308 |
1 |
|
|
|
|
|
|
|
|
|
|
5 |
r = -.438* |
r = -.372 |
r = -.120 |
r = -.120 |
1 |
|
|
|
|
|
|
|
|
|
6 |
r = -.153 |
r = .005 |
r = .279 |
r = .284 |
r = .621** |
1 |
|
|
|
|
|
|
|
|
7 |
r = -.393 |
r = -.208 |
r = -.102 |
r = .166 |
r = .557** |
r = .400 |
1 |
|
|
|
|
|
|
|
8 |
r = -.399 |
r = -.358 |
r = -.251 |
r = .403 |
r = .294 |
r = .454* |
r = .551* |
1 |
|
|
|
|
|
|
9 |
r = -.177 |
r = -180 |
r = -.145 |
r = .195 |
r = .379 |
r = .217 |
r = .296 |
r = .366 |
1 |
|
|
|
|
|
10 |
r = .308 |
r = -.011 |
r = .050 |
r = -.389 |
r = -.363 |
r = -.271 |
r = -.332 |
r = -.244 |
r = -.022 |
1 |
|
|
|
|
11 |
r = .229 |
r = .203 |
r = .151 |
r = -.202 |
r = -.384 |
r = -.263 |
r = .033 |
r = -.216 |
r = -.339 |
r = .332 |
1 |
|
|
|
12 |
r = .234 |
r = -.228 |
r = -.148 |
r = .157 |
r = .051 |
r = .209 |
r = -.091 |
r = .115 |
r = .114 |
r = .033 |
r = .046 |
1 |
|
|
13 |
r = -.034 |
r = -.207 |
r = .082 |
r = .051 |
r = .128 |
r = .070 |
r = .080 |
r = .418 |
r = .457* |
r = -.382 |
r = -.359 |
r = .418 |
1 |
|
*indicates significance at the 0.05 level **indicates significance at the 0.01 level 1. CVLT-II List A Immediate Free Recall Trials 1–5; 2. CVLT-II List A Long-Delay Free Recall; 3. CVLT-II List A Long-Delay Yes/No Recognition; 4. CVLT-II List A Long-Delay Yes/No Recognition False-Positives; 5. D-KEFS TMT: Visual Scanning; 6. D-KEFS TMT: Number Sequencing; 7. D-KEFS TMT: Letter Sequencing; 8. D-KEFS TMT: Number-Letter Switching; 9. D-KEFS TMT: Motor Speed; 10. D-KEFS VFT: Letter Fluency; 11. D-KEFS VFT: Category Fluency; 12. D-KEFS VFT: Category Switching; 13. Encephalapp Stroop Test: Stroop On |
|||||||||||||
Discussion
The purpose of this study was to examine whether older adults with SCD would benefit from Perceptual-Cognitive Training. The results indicate a significant difference between treatment and control groups in tasks of episodic memory, working memory, cognitive flexibility and processing speed. After the 14 sessions of brain stimulation with PCT (T2) the treatment group performed better compared to controls in a task of episodic memory, such as retrieving the previous encoded abstract wordlist after a long delay (CVLT-II List A/B LDFR), and in a task of cognitive flexibility, such as generating words by alternating between two categories (D-KEFS VF CS). Furthermore, a trend of higher performance was noticed in the treatment group in another task of episodic memory, immediate free recall CVLT-II List A/B IFR).
One month follow-up after the Perceptual-Cognitive Training (T3), the benefits observed for the participants of the treatment group in retrieving words after a long delay were maintained and were significantly higher compared to controls. Furthermore, a significant major effect between groups was observed in others episodic memory tasks such as immediate free recall, (CVLT-II List A/B IFR) and short delay recall (CVLT-II List A/B SDFR). A significant major effect after a month follow-up was observed in treatment participants in a verbal cognitive flexibility task (D-KEFS VF CS) and a trend of higher performance was noticed in a visual cognitive flexibility task (D-KEFS TMT: NLS). Furthermore, the treatment group performed significantly better in a working memory task, such as repeating digits backward (Digit Span Backward) and showed a trend of better scores in Total Digit Span. Similarly, the treatment group performed better compared to controls in tasks of processing speed (D-KEFS TMT: LS; D-KEFS VF LF). Moreover, a trend of higher performance was noticed in the treatment group compared to controls in the accuracy and the number of words recognized from a bigger list after a long delay (CVLT-II List A/B LDR FPE). Specifically, the participants of the control group reported a greater number of false-positive errors after seven and twelve weeks of follow-up.
Previous studies have demonstrated that computerized cognitive training programs serve as powerful tools to enhance cognition in healthy older adults [18, 22, 23, 30]. The current study expands on these findings by showing additional benefits of computer training on cognition in older adults with subjective cognitive decline. Similar benefits in memory, processing speed, working memory and cognitive flexibility were found in previous studies on PCT intervention [33, 37, 60 ] in different populations (e.g. healthy young adults and students with neurodevelopmental conditions, healthy adults and adults with concussions, healthy older adults and older adults with subjective memory complaints). For example, a case study on an 80-year-old man with memory complaints, that underwent 32 sessions of training with PCT, showed improvements in working memory, episodic memory, processing speed, as well as reduction in cognitive complaints with positive impact on quality of life. Other work from our laboratory on healthy older adults indicated improvements in cognitive flexibility after just 7 sessions (i.e. 21 trials) of PCT [34]. Parsons et al., [33] found that students who performed 10 sessions of PCT improved in performance as investigated with standardized cognitive assessments of working memory and attention on visual information. Tullo et al., [37] observed that performing 15 sessions of PCT was associated with increased attentional abilities in students with neurodevelopmental conditions (e.g. Autism Spectrum Disorder, Attention-Deficit/Hyperactivity Disorder, Intellectual Disability, Specific Learning Disorder. Similarly, etc.). Vartanian and colleagues [60] trained military personal with the PCT and observed improved performances on working memory task compared to no improvements from participants who underwent PCT training.
Considerable evidence [6, 61, 62,], from both behavioral and neurobiological sources, suggests that age-related memory declines might be linked to deficits in executive functioning (EF), including inhibitory functions, working memory [63,64], and cognitive flexibility [4, 64, 65,]. Memory tasks involve the organization of new information, selective attention for the information that has to be encoded, the suppression of unnecessary information, and at times the maintenance and shifting of cognitive sets, so this is not surprising in many ways. Furthermore, in order to encode and retrieve new information cognitive efficiency relies on processing speed and working memory. Evidence suggests that slow processing speed or working memory difficulties [9, 66] in older adults impact on the accuracy of encoding new information and on the retrieval of it later on [9, 66]. This pattern of deficits in executive tasks associated with episodic memory decline is consistent with the view that underlying cognitive functions depend on multiple-interacting neural networks, including the medial temporal memory complex and prefrontal cortical executive system [67, 68]. Therefore, any memory enhancement obtained after PCT may be in part due to improvements in processing speed, working memory (i.e. brief sustained attention), and cognitive flexibility. The treatment group became significantly faster in processing new information, such as word production or connecting letters with distractor numbers presented on the page, faster in tasks that require certain mental flexibility, and better in encoding and retrieving an abstract wordlist after a short and long delay. The enhancement in these cognitive tasks was also associated with a significant correlation between improved processing speed and the performance in memory tasks. In contrast, we observed that the control group was slower in processing speed and retrieved fewer words compared to the treatment group. Further, no significant relationship between processing speed and memory task performance was observed in the control group. These findings are interesting and require further replication, possibly with the inclusion of a second control group of healthy older adults.
Consistent with some imaging studies, episodic memory functioning is the most robust neuropsychological predictor of dementia [69–71]. One recent study found that performance for immediate versus delayed episodic memory recall varies according to the temporal stage of disease progression [30, 72]. Contrary to the common view that delayed memory recall is the most sensitive measure of early dementia, Bilgel et al. found that immediate verbal recall measures in the CVLT were the first to decline in preclinical dementia, followed by delayed verbal recall on the same test closer to a diagnosis of mild cognitive impairment. Although research on PCT does not typically result in generalization of learning to daily living tasks in older adults [33, 37, 54, 73], an interesting result observed in our study is the transfer effect between PCT and episodic memory tasks. For example, the older adults with SCD from the treatment group showed a significant enhancement in episodic memory tasks such as learning abstract word lists and retrieving words after a short and a long delay period (e.g. 30 min). Although the benefits on memory tasks have no overlap with the trained cognitive functions of PCT and may thus be considered a far transfer [74, 75], this transfer was characterized by a medium-large effect size and a power above .80. This reflects the effectiveness of PCT, though little is known about the transfer effect between the PCT and memory performance in older adult with SMCs. That being said, PCT intervention may play a significant role in dementia prevention or cognitive decline but further research is needed to ensure reliability and validity. The concept of adult neurogenesis provides an interesting potential mechanism for the cognitive benefits observed in the treatment group, particularly since benefits were still observed in the follow-up testing a month later. Here the hypothesis would be that the PCT provides enough cognitive enrichment to enhance adult neurogenesis. This is similar to the effect observed in animals that exercise or are in enriched environments [76], which rely on increases in neurotrophin levels [77, 78]. Indeed, learning behaviours that involve the hippocampus have been shown to impact adult neurogenesis in animal models [79].
An increasing number of studies have examined how environmental and/or behavioural factors can modulate neurogenesis and subsequently effect hippocampal-dependent learning and memory in humans [80]. Indeed, exercise has even been shown to be beneficial for individuals with subjective memory complaints, enhancing medial temporal lobe thickness [81]. The time course for the increase in performance observed one month after testing corresponds well with the time course for new neurons generated in response to the PCT training to be incorporated into, and enhance, existing networks [82]. In addition, an increased activation of the neural structures and circuits was observed during PCT training in a recent fMRI investigation [34]. These neural areas are involved in executive function tasks. Thus it would be interesting for future studies to determine if PCT has the capacity to promote neuroplasticity, providing a mechanism through which it can enhance learning and memory processes [83].
A very positive aspect of the PCT intervention was the ability of older adults to be able to engage in this computerized training task, even if their performance was slower than in younger adult groups [84, 85]. The learning curve in our study indicates that PCT can be a good cognitive training tool for older populations. Moreover, as PCT involves an individualized dynamic and homeostatic adjustment of the training speed, the subjects found they could easily work with the program irrespective of their initial performance. Because each trial was based upon the participant’s performance in the prior trial, the software provided a continuous challenge that helped maintain a high level of engagement and motivation. Hence, participants can remain highly motivated to engage regularly in the training regimen. Therefore, the results should be replicated by further research on clinical older population to ensure reliability.
Limitations
The use of an inactive control group does not exclude the possibility that this empirical finding reflects a placebo effect [86], although, a greater significant difference in cognitive performance was observed between groups not only after PCT intervention but also at the second follow-up (T3), where both groups were on rest for 4 weeks (i.e. no intervention was administered). Therefore, the results should be replicated by further research to ensure reliability. A limitation of this study was the non-administration of the memory complaint rating scale (MAC-Q) after the PCT intervention (i.e. MAC-Q was only used to assess the inclusion/exclusion criteria of this study). In addition, research would benefit from using a quality of life questionnaire test to assess the transfer of these cognitive benefits on daily activities.
Conclusions
The current study demonstrated improved performance in older adults with SCD on measures of episodic memory, processing speed, working memory, and cognitive flexibility. The prolonged enhancement result observed over a month may hold promise for cognitive rehabilitation/neurogenesis, but it needs to be replicated to further support its validity, in both healthy samples and those with neurocognitive disorders or types of dementia. Further research is essential to examine structural neuroplasticity and transfer effects from the PCT to daily tasks. Taken together, the results of this study suggest that the PCT may be an effective tool for cognitive enhancement in preclinical and clinical populations of older adults.
Acknowledgement
We would like to thank all participants for their significant commitment to this study. Thank you also to the Christie Lab graduate and undergraduate students who assisted with data collection. A special thank you is reserved for Dr. Scott Hofer and the Institute on Aging and Lifelong Health. SM was supported by the Fondazione Banca del Monte di Lombardia for travel to Canada to conduct this research. The protocol can be accessed on Clinical.trials.gov with the following registry number: NCT03763344.
References
- WHO (2016) Ageing.
- Suzman R, Beard J (2011) Global Health and Aging. NIH Publication No 117737, 1: 273–277. https://doi.org/11-7737
- De Grey AD, Ames BN, Andersen JK, Bartke A, Campisi J et al. (2002) Time to talk SENS: critiquing the immutability of human aging. Ann N Y Acad Sci 959: 452–462.
- Kehagia AA, Murray GK, Robbins TW (2010) Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Current Opinion in Neurobiology 20: 199–204.
- Salthouse TA (2013) Effects of age and ability on components of cognitive change. Intelligence 41: 501–511.
- Salthouse TA, Atkinson TM, Berish DE (2003) Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology. General 132: 566–594.
- Dixon RA (2009) An epidemiological approach to cognitive health in aging. In Memory, Aging and the Brain: A Festschrift in Honour of Lars-Göran Nilsson. https://doi.org/10.4324/9780203866665
- Rabin LA, Smart CM, Amariglio RE (2017) Subjective Cognitive Decline in Preclinical Alzheimer’s disease. Annual Review of Clinical Psychology 13: 369–396.
- Goldberg E (2009) The new executive brain: Frontal lobes in a complex world. The New Executive Brain: Frontal Lobes in a Complex World. Journal of Economic Psychology 32: 688–690.
- Reuter-Lorenz PA, Park DC (2010) Human Neuroscience and the Aging Mind: A New Look at Old Problems. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences 65B: 405–415.
- Craik FIM (2016) A functional account of age differences in memory. In Memory, Attention, and Aging: Selected Works of Fergus I. M. Craik, Routledge, New York Pg No: 340.
- Tulving E, Stevenson RJ, Boakes RA (2002) Episodic memory: from mind to brain\rA mnemonic theory of odor perception. Annu Rev Psychol
- McDowd JM, Shaw RJ (2000) Attention and aging: A functional perspective. In F. I. M. Craik & T. A. Salthouse (Eds.), The handbook of aging and cognition (p. 221–292). Lawrence Erlbaum Associates Publishers.
- Craik FIM, Byrd M (1982) Aging and Cognitive Deficits. In Aging and Cognitive Processes 191–211.
- Salthouse TA (1996) The Processing-Speed Theory of Adult Age Differences in Cognition. Psychological Review 103: 403–428.
- Hasher L, Zacks RT (1988) Working memory, comprehension, and aging: A review and a new view BT – The psychology of learning and motivation. The Psychology of Learning and Motivation 22: 193–225.
- Bherer L (2015) Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Annals of the New York Academy of Sciences 1337: 1–6.
- Kueider AM, Parisi JM, Gross AL, Rebok GW (2012) Computerized cognitive training with older adults: A systematic review. PLoS ONE 7.
- Straubmeier M, Behrndt EM, Seidl H, Özbe D, Luttenberger K et al. (2017) Non-Pharmacological Treatment in People With Cognitive Impairment. Deutsches Aerzteblatt Online 114: 815–821.
- Teixeira CVL, Gobbi LTB, Corazza DI, Stella F, Costa JLR et al. (2012) Non-pharmacological interventions on cognitive functions in older people with mild cognitive impairment (MCI). Archives of Gerontology and Geriatrics 54: 175–180.
- Garner J (2009) Conceptualizing the relations between executive functions and self-regulated learning. Journal of Psychology 143: 405–426.
- Hampstead BM, Sathian K, Phillips PA, Amaraneni A, Delaune WR et al. (2012) Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: A randomized, single-blind study. Neuropsychology 26: 385–399.
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F et al. (2013) Video game training enhances cognitive control in older adults. Nature 501: 97–101.
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB et al. (2010) The influence of perceptual training on working memory in older adults. PLoS ONE 5: 1–8.
- Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K et al. (2006) Testing the limits of cognitive plasticity in older adults: Application to attentional control. ActaPsychologica 123: 261–278.
- Edwards JD, Wadley VG, Vance DE, Wood K, Roenker DL (2013) The impact of speed of processing training on cognitive and everyday performance. Aging & Mental Health 9: 37–41.
- Mozolic JL, Long AB, Morgan AR, Rawley-Payne M, Laurienti PJ (2011) A cognitive training intervention improves modality-specific attention in a randomized controlled trial of healthy older adults. Neurobiology of Aging 32: 655–668.
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD et al. (2002) Effects of cognitive training interventions with older adults: A randomized controlled trial. Journal of the American Medical Association. 288: 2271–2281.
- Kramer AF, Larish JL, Weber TA, Bardell L (1999) Training for executive control: Task coordination strategies and aging. Attention and Performance.
- Mortamais M, Ash JA, Harrison J, Kaye J, Kramer J et al. (2017) Detecting cognitive changes in preclinical Alzheimer’s disease: A review of its feasibility. Alzheimer’s and Dementia 13: 468–492.
- Assed MM, de Carvalho MKHV, Rocca CC de A, Serafim A de P (2016) Memory training and benefits for quality of life in the elderly: A case report. Dementia & Neuropsychologia 10: 152–155.
- Cavanagh P, Alvarez GA (2005) Tracking multiple targets with multifocal attention. TRENDS in Cognitive Sciences 9: 349–354.
- Parsons B, Magill T, Boucher A, Zhang M, Zogbo K et al. (2016) Enhancing Cognitive Function Using Perceptual-Cognitive Training. Clinical EEG and Neuroscience 47: 37–47.
- Spaner CR, Musteata S, Kenny RA, Gawryluk JR, Hofer S et al. (2019) 3-Dimensional Multiple Object Tracking Training Can Enhance Selective Attention , Psychomotor Speed , and Cognitive Flexibility in Healthy Older Adults. Ageing Sci Ment Health Stud 3: 1–12.
- Bottari C, Dassa CM, Rainville C, Dutil ÉL (2009) The criterion-related validity of the IADL Profile with measures of executive functions, indices of trauma severity and sociodemographic characteristics. Brain Injury 23: 322–335.
- Corbin-Berrigan LA, Kowalski K, Faubert J, Christie B, Gagnon I (2018) Three-dimensional multiple object tracking in the pediatric population: the NeuroTracker and its promising role in the management of mild traumatic brain injury. NeuroReport 29: 559–563.
- Tullo D, Guy J, Faubert J, Bertone A (2018) Training with a three-dimensional multiple object-tracking (3D-MOT) paradigm improves attention in students with a neurodevelopmental condition: a randomized controlled trial. Developmental Science 21.
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12: 189–198.
- Mahoney FI, Barthel DW (1965) Functional Evaluation: the Barthel Index. Maryland State Medical Journal 14: 61–65.
- Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist 9: 179–186.
- Crook III TH, Feher EP, Larrabee GJ (1992) Assessment of memory complaint in age-associated memory impairment: the MAC-Q. International Psychogeriatrics 4: 165–176.
- Yesavage Ja, Brink TL, Rose TL, Lum O, Huang V et al. (1983) Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research 17: 37–49.
- Pachana NA, Byrne GJ, Siddle H, Koloski N, Harley E et al. (2007) Development and validation of the Geriatric Anxiety Inventory. International Psychogeriatrics 19: 103–114.
- Zokaei N, Mackellar C, Čepukaitytė G, Patai EZ, Nobre AC (2017) Cognitive training in the elderly: Bottlenecks and new avenues. Journal of Cognitive Neuroscience 29: 1473–1482.
- Cooper DB, Epker M, Lacritz L, Weine M, Rosenberg RN et al. (2001) Effects of practice on category fluency in Alzheimer’s disease. The Clinical Neuropsychologist 15: 125–128.
- Cooper DB, Lacritz LH, Weiner MF, Rosenberg RN, Cullum CM (2004) Category fluency in mild cognitive impairment: Reduced effect of practice in test-retest conditions. Alzheimer Disease and Associated Disorders 18: 120–122.
- Woods SP, Delis DC, Scott JC, Kramer JH, Holdnack JA (2006) The California Verbal Learning Test – second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology 21: 413–420.
- Delis DC, Kramer JH, Kaplan E, Ober BA (2000) California Verbal Learning Test. 2nd edn. Adult version. Manual. Test.
- Delis, D, Kaplan E, Kramer J (2001) Delis-Kaplan executive function system (D-KEFS). Canadian Journal of School Psychology 20: 117–128.
- Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB et al. (2016) Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. American Journal of Gastroenterology 111: 78–86.
- Bajaj JS, Heuman DM, Sterling RK, Sanyal AJ, Siddiqui M et al. (2015) Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clinical Gastroenterology and Hepatology 13: 1828–1835.
- Stroop JR (1935) Stroop color word test. J. Exp. Physiol
- Faubert J (2002) Visual perception and aging. Can J Exp Psychol 56: 164–176.
- Faubert J, Sidebottom L (2013) NeuroTracker System: Its role for perceptual-cognitive training of athletes and its potential impact on injury reductions and concussion management in sports. Journal of Clinical Sports
- Faubert J, Sidebottom L (2012) Perceptual-Cognitive Training of Athletes. Journal of Clinical Sport Psychology 6: 85–102.
- Van Merriënboer JJG, Sweller J (2010) Cognitive load theory in health professional education: design principles and strategies. Medical Education 44: 85–93.
- Hairston WD, Maldjian JA (2009) An adaptive staircase procedure for the E-Prime programming environment. Computer Methods and Programs in Biomedicine 93: 104–108.
- Field A (2013) Moderation, mediation and more regression. Discovering Statistic Using SPSS 4th (edn).
- Yantis S (2002) Stevens’ handbook of experimental psychology. Stephens’ Handbook of Experimental Psychology. https://doi.org/10.1026//1618-3169.50.2.155.
- Vartanian O, Coady L, Blackler K (2016) 3D multiple object tracking boosts working memory span: Implications for cognitive training in military populations. Military Psychology 28: 353–360.
- Glisky EL, Polster MR, Routhieaux BC (1995) Double Dissociation Between Item and Source Memory. Neuropsychology 9: 229–235.
- Hedden T, Gabrieli JDE (2004) Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience 5: 87–96.
- Johnson MK, Reeder Ja, Raye CL, Mitchell KJ (2002) Second thoughts versus second looks: an age-related deficit in reflectively refreshing just-activated information. Psychological Science : A Journal of the American Psychological Society / APS 13: 64–67.
- Mitchell KJ, Johnson MK, Raye CL, Greene EJ (2004) Prefrontal cortex activity associated with source monitoring in a working memory task. Journal of Cognitive Neuroscience 16: 921–934.
- Spiro RJ, Collins BP, Thota JJ, Feltovich PJ (2003) Cognitive Flexibility Theory: Hypermedia for Complex Learning, Adaptive Knowledge Application, and Experience Acceleration. Educational Technology 43: 5–10.
- Salthouse TA (1994) Aging associations: influence of speed on adult age differences in associative learning. Journal of Experimental Psychology Learning Memory and Cognition 20: 1486–1503.
- Abutalebi J, Green DW (2016) Neuroimaging of language control in bilinguals: Neural adaptation and reserve. Bilingualism 19: 689–698.
- Craik FIM, Bialystok E (2006) Cognition through the lifespan: Mechanisms of change. Trends in Cognitive Sciences 10: 131–138.
- Beck IR, Gagneux-Zurbriggen A, Berres M, Taylor KI, Monsch AU (2012) Comparison of verbal episodic memory measures: Consortium to establish a registry for alzheimer’s disease-neuropsychological assessment battery (CERAD-NAB) versus California verbal learning test (CVLT). Archives of Clinical Neuropsychology 27: 510–519.
- Hodges JR, Graham KS (2001) Episodic memory: insights from semantic dementia. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 356: 1423–1434.
- Peter J, Scheef L, Abdulkadir A, Boecker H, Heneka M et al. (2014) Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimer’s and Dementia 10: 99–108.
- Bilgel M, An Y, Lang A, Prince J, Ferrucci L et al. (2014) Trajectories of Alzheimer disease-related cognitive measures in a longitudinal sample. Alzheimer’s and Dementia 10: 735–742.
- Legault I, Allard R, Faubert J (2013) Healthy older observers show equivalent perceptual-cognitive training benefits to young adults for multiple object tracking. Frontiers in Psychology 4: 323.
- Klahr D, Chen Z (2011) Finding One’s place in transfer space. Child Development Perspectives 5: 196–204.
- Zelinski EM (2009) Far transfer in cognitive training of older adults. Restorative Neurology and Neuroscience 27: 455–471.
- Patten AR, Yau SY, Fontaine CJ, Meconi A, Wortman RC et al. (2015) The Benefits of Exercise on Structural and Functional Plasticity in the Rodent Hippocampus of Different Disease Models. Brain Plasticity: 1: 97–127.
- Boehme F, Gil-Mohapel J, Cox A, Patten A, Giles E et al. (2011) Voluntary exercise induces adult hippocampal neurogenesis and BDNF expression in a rodent model of fetal alcohol spectrum disorders. European Journal of Neuroscience 33: 1799–1811.
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH et al. (2004) Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 124: 71–79.
- Epp JR, Chow C, Galea LAM (2013) Hippocampus-dependent learning influences hippocampal neurogenesis. Frontiers in Neuroscience 7: 57.
- Bruel-Jungerman E, Rampon C, Laroche S (2007) Adult hippocampal neurogenesis, synaptic plasticity and memory: facts and hypotheses. Reviews in the Neurosciences 18: 93–114.
- Siddarth P, Rahi B, Emerson ND, Burggren AC, Miller KJ et al. (2018) Physical activity and hippocampal sub-region structure in older adults with memory complaints. Journal of Alzheimer’s Disease 61: 1089–1096.
- Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD et al. (2002) Functional neurogenesis in the adult hippocampus. Nature 415: 1030–1034.
- Sörman DE, Rönnlund M, Sundström A, Adolfsson R, Nilsson LG (2015) Social relationships and risk of dementia: A population-based study. International Psychogeriatrics 27: 1391–1399.
- Rush B, Barch D, Braver T (2006) Accounting for cognitive aging: Context processing, inhibition or processing speed? Aging, Neuropsychology, and Cognition 13: 588–610.
- Trick LM, Perl T, Sethi N (2005) Age-related differences in multiple-object tracking. Journals of Gerontology – Series B Psychological Sciences and Social Sciences 60: 102–105.
- Boot WR, Simons DJ, Stothart C, Stutts C (2013) The Pervasive Problem With Placebos in Psychology: Why Active Control Groups Are Not Sufficient to Rule Out Placebo Effects. Perspectives on Psychological Science 8: 445–454.