Abstract
Purpose: To assess the relationship between IFN-γR expression and clinical radiotherapy outcome in nasopharyngeal carcinoma.
Methods: IFN-γR expression was examined by immunohistochemistry from 70 nasopharyngeal carcinoma patients. Then, statistical analysis was conducted to explore the correlation between IFN-γR expression and tumor clinicopathological charateristics. Finally, CNE-1 cells were employed to detect the effects of IFN-γR expression on radiotherapy outcomes by in vitro assays.
Results: IFN-γR was upregulated dramatically in nasopharyngeal carcinoma tissues. Elevated expression of IFN-γR correlated significantly with tumor stage, positive lymph node metastasis and poor prognosis in patients with nasopharyngeal carcinoma. In vitro assays demonstrated that overexpression of IFN-γR promoted the radio-resistance ability in CNE-1 cells. Knockdown of IFN-γR facilitated CNE-1 cells more sensitive to radiation, and a wobble mutant of IFN-γR could restore this effect.
Conclusions: Overexpression of IFN-γR may correlate positively with radiotherapy resistance of nasopharyngeal carcinoma.
Keywords
IFN-γR; overexpression; prognosis; radiation resistance; NPC
Introduction
Nasopharyngeal carcinoma is one of the most common malignancies and occupies the seventh leading cause of cancer-related deaths in China [1]. Particularly in South China, nasopharyngeal carcinoma is the most prevalent. Locally, advanced nasopharyngeal carcinoma patients are generally treated with radiotherapy, and the prognosis is related with radiotherapy [2]. Both local-regional failure and early systemic dissemination of the disease always contribute to treatment failure, which makes radio-sensitization promising in improving the outcome of therapeutic irradiation [3].
IFN-γ plays a pleiotropic role in modulating immunity and antiviral activity. It stimulates macrophages and NK cells to produce MHC class I and II molecules, while IFN-γR is not required for the development of the immune system [4]. Researches showed that IFN-γR was overexpressed in breast cancer and its amplification was closely related with lymph node metastasis and poor prognosis [5-7]. Until now, there are still no reports about whether IFN-γR has an effect on the radiotherapy for NPC. In the present study, we found for the first time that IFN-γR expression correlated with the prognosis of NPC patients who received radiotherapy. High IFN-γR levels improved the radiation resistance ability of NPC cells.
Patients and methods
Tissue samples
Seventy tissues of nasopharyngeal carcinoma were freshly collected by nasopharyngoscope and procured by the Department of Pathology, Cancer Center of Wuhan Union Hospital, China. The collection of tumor samples was approved by the institutional ethics committee. Informed consent forms had been signed for sample collection before. Tumor regions were technically separated by experienced pathologists and promptly stored at liquid nitrogen until needed. All patients included underwent concurrent chemotherapy (Cisplatin, 40mg/m2, weekly) with radiation therapy for the first time. They received irradiation with daily fraction doses of 2.12Gy for a total dose 70 Gy, which was administered with linear accelerators. The clinicopathologic features of patients were showed in Table 1.
Immunohistochemical staining
Immunohistochemical staining was conducted as previously described. After rehydration, the paraffin-embedded nasopharyngeal carcinoma tissues were immersed in 3% hydrogen peroxide solution for 10 min, and then heated in EDTA buffer (pH 8.0) for 25 min. The slides were blocked with 10% normal rabbit serum at 37°C for 30 min, and then incubated with rabbit polyclonal antibody against primary antibody (IFN-γR, 1:200, ABCAM) overnight at 37°C. The slides were washed with PBS, and then incubated with biotinylated second antibody (diluted 1:200) for another 30 min at 37°C. Finally, streptavidin-peroxidase reactivity was detected with DAB solution. Cell nucleus was counterstained with hematoxylin. The immunohistochemical slides were graded according to the ratio of tumor cells with nuclear labeling. The level of IFN-γR- positive staining was scored into three grades: 0 for < 10%, positive for 10–30%, strong positive for > 30%.
Plasmid constructs and small interfering RNA synthesis
Double-stranded oligonucleotide with the following sequence was synthesized into the pSilencer 3.1- H1 neo small interfering RNA (siRNA) expression vector, and the scrambled siRNA was applied as control. Mutant DNA-IFN-γR (BOSTER, Wuhan, China) was used to resist the effect of IFN-γR-RNAi.
Cell culture and transfection
The CNE-1 cells (one of the esophageal squamous carcinoma cell lines) were cultured in RPMI 1640 (Invitrogen) with 10% fetal bovine serum. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instruction. Cells were harvested after transient transfection for 48 h. For stable transfection, cells were selected with 200 μg/ml G418, and passaged by serial dilution. During the next two weeks, colonies were screened by selective medium. The positive colonies with IFN-γR overexpression and IFN-γR knockdown were chosen for experiments.
Western blot analysis
Total proteins were isolated from patient tissues and cultured cells with lysis buffer [10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP40] containing protease inhibitors. After centrifugation (15,000 RCF, 30 min, 4°C), supernatants were recovered for immunoblot analysis. The proteins were subjected to SDS-PAGE and then transferred onto polyvinylidenedifluoride (PVDF) membranes (Millipore). Next, the membranes were blocked and then incubated with primary antibody against IFN-γR (1:500, ABCAM) at 4°C overnight, then incubated with horseradish peroxidase-conjugated accordingly secondary antibody for 1 h at room temperature and developed using ECL detection reagent (BOSTER).
Ionizing radiation
CNE-1 cell line was exposed to IR in a JL Shepherd Model 143 137Cesium gamma and irradiated at a rate of 2.4 Gy/min.
Colony formation assay
A total of 400 cells were aliquoted into 6- well plate and exposed to γ rays in a dose of 5 Gy in triplicate. After incubation for 10 days, visible colonies were fixed in methanol and stained with Giemsa. Colonies were counted in each well. Each experiment was performed three times independently.
Results
Overexpression of IFN-γR in esophageal squamous cell carcinoma
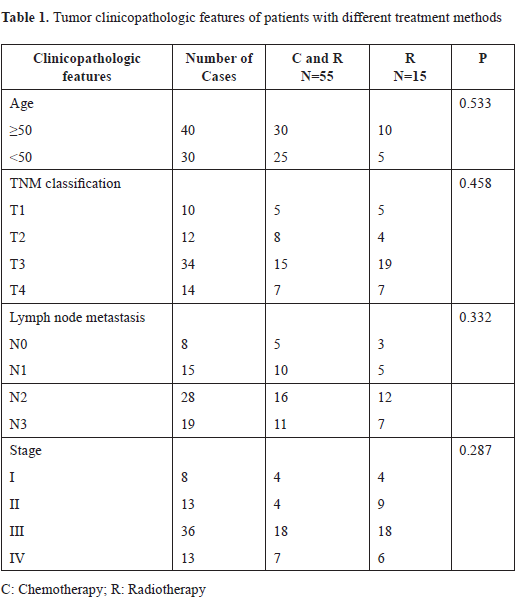
IFN-γR expression was examined in 70 NPC samples and adjacent normal tissue by immunohistochemistry. Results showed that IFN-γR was hardly expressed or revealed weak expression in normal epithelial cells. While, in 36 cases of 70 NPC samples, IFN-γR expression exhibited significantly enhanced trend compared to the normal epithelium [Table 1]. Statistical analysis indicated that elevated expression of IFN-γR was significantly related to tumor stage, clinical stage and lymph node metastasis (p < 0.05) [Table 2].
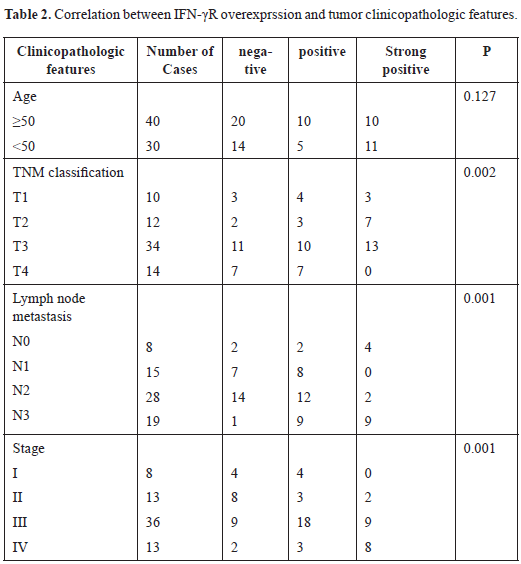
Next, the effect of IFN-γR expression on patient’s survival was analyzed. IFN-γR expression is positive in some patients and negative positive in others [Figure 1A]. After the included patients underwent chemotherapy combined with radiation therapy (55 out of 70), compared to NPC patients with low IFN-γR expression in cancerous tissue was significantly higher, patients with cancerous tissue of high IFN-γR expression showed significantly lower survival rate (p = 0.001) [Figure 1B].
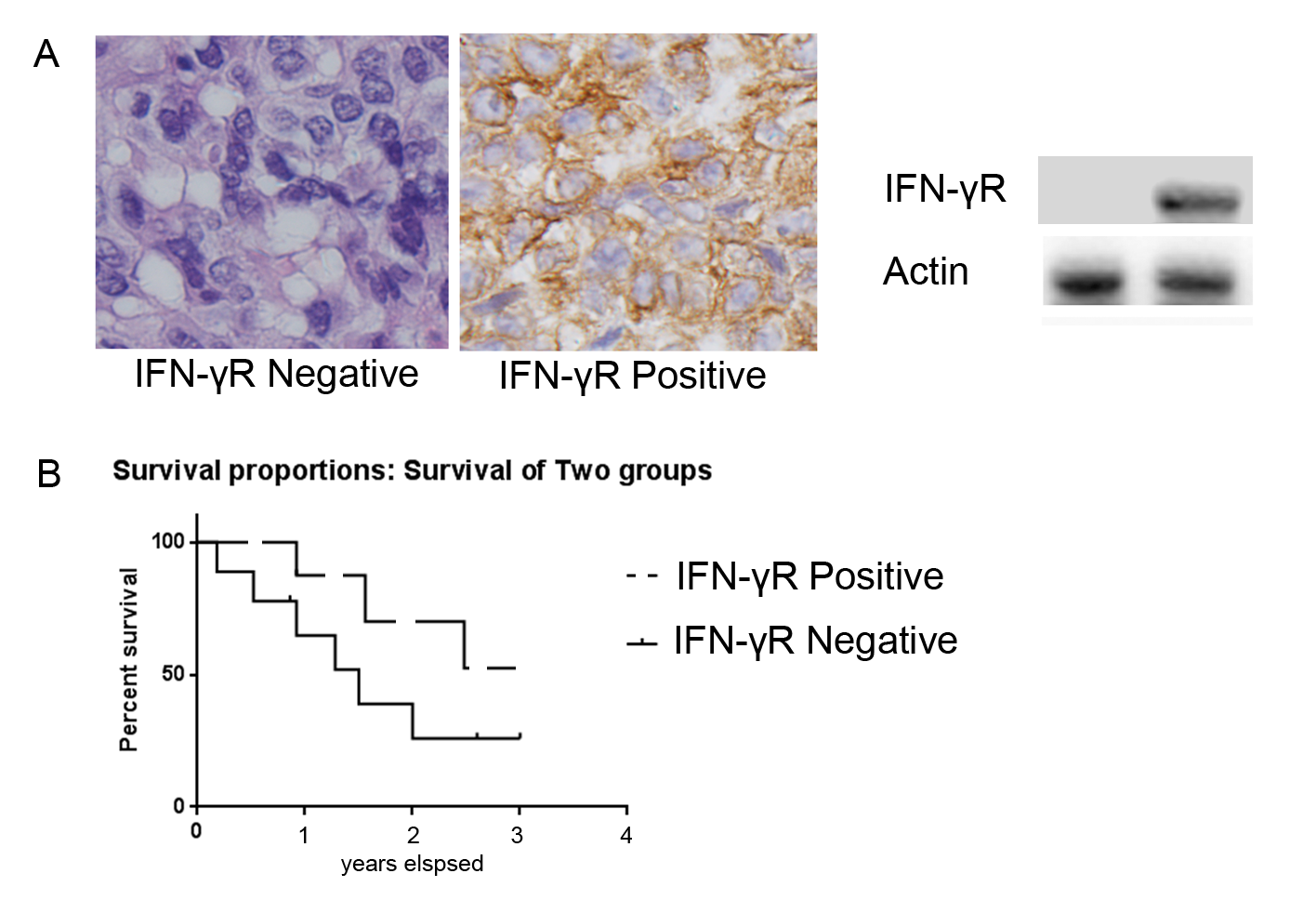
Figure 1. Representative IFN-γR immunostaining in NPC and effects of IFN-γR expression on patient’s survival. A: IFN-γR expression is positive in some patients and negative positive in others. B: In the patients that received concurrent chemotherapy with radiation therapy (55 out of 70), the survival rate of patients with low IFN-γR expression was significantly higher than that of patients with high IFN-γR expression (p = 0.001).
Effects of IFN-γR on the radio-sensitivity of CNE-1 cells
To confirm the clinical data, CNE-1 cells were radiated with a dose of 5 Gy for 0, 2, 4, 8 and 12 h to know IFN-γR expression change. IFN-γR expression was increased after radiation in a time-dependent manner, which implied that IFN-γR may be associated with the radiation response of CNE-1 cells [Figure 2].
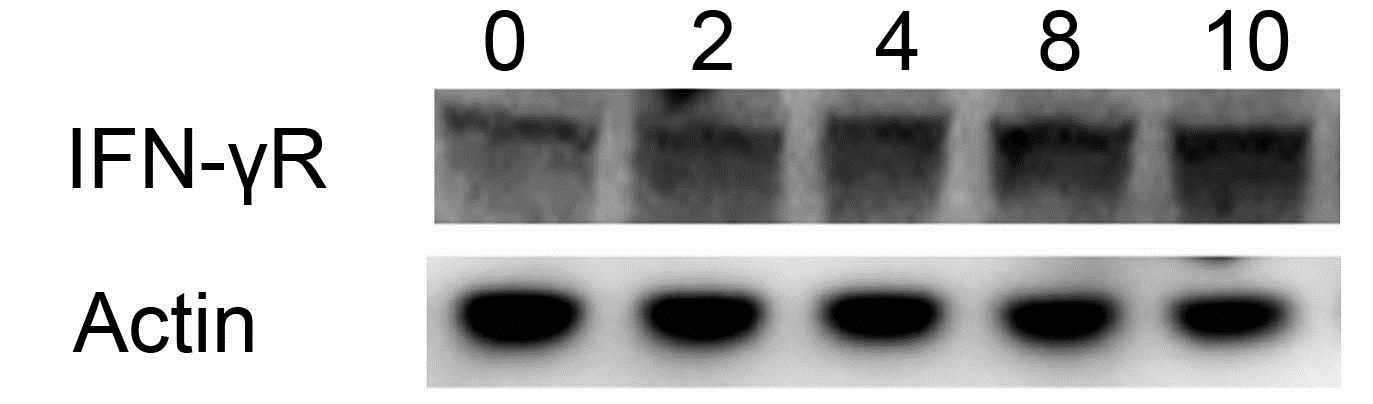
Figure 2. Expression of IFN-γR induced by radiation in CNE-1 cells. Elevated IFN-γR was observed after γ ray radiation with a dose of 5 Gy, and its level was highest at 10 hours.
Colony formation assay was conducted to detect the effects of IFN-γR on the radio-sensitivity of CNE-1 cells [8]. IFN-γR knock-down cells showed a significantly lower survival fraction than control groups [Figures 3A and B]. Conversely, the survival fraction of cells with forced IFN-γR expression was higher [Figures 3C and D]. Therefore, results indicated a positive relationship between IFN-γR expression and radio-sensitivity of CNE-1 cells.
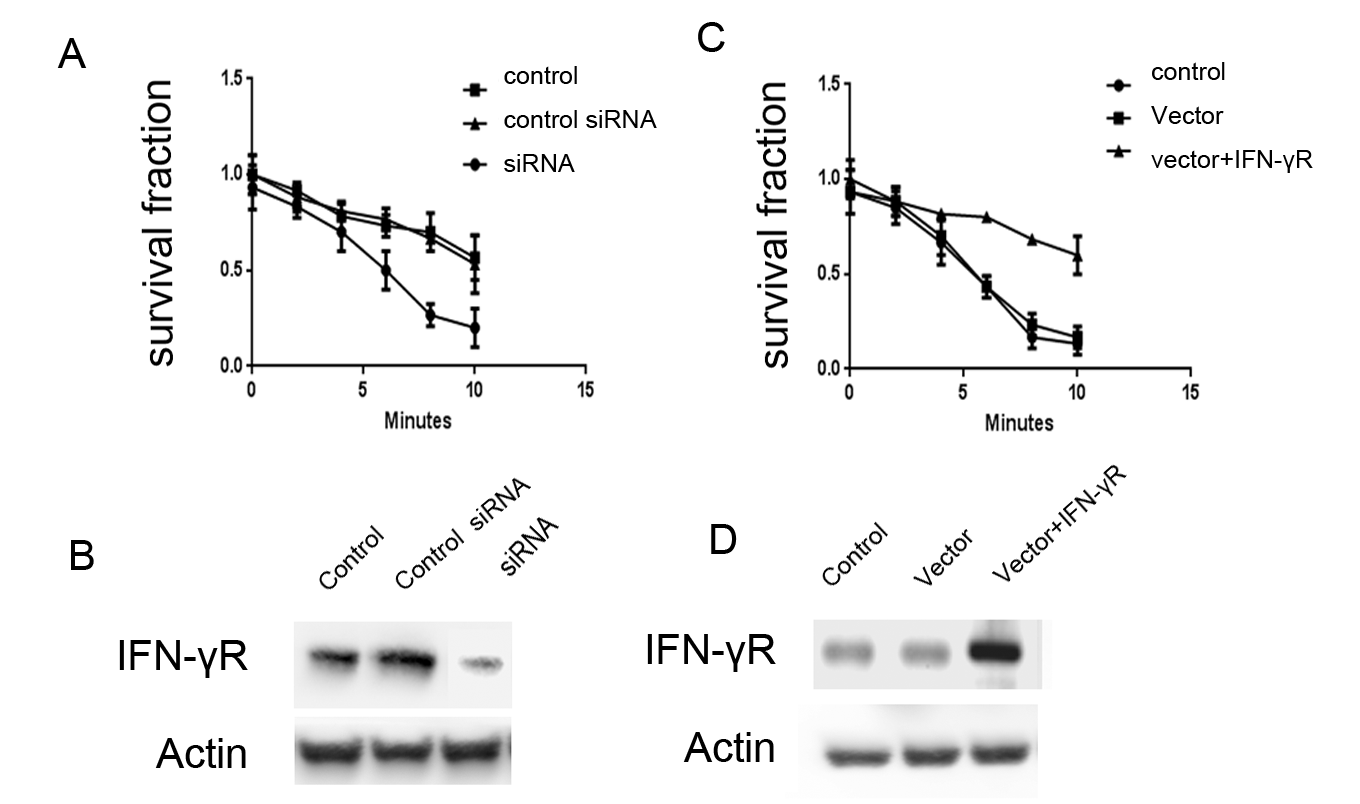
Figure 3. Effects of IFN-γR expression on the radio-sensitivity of CNE-1 cells. A: Expression of IFN-γR decreased in IFN-γR RNAi cells. B: Knock-down of IFN-γR promoted the radio-sensitivity of CNE-1 cells. C: Expression of IFN-γR increased after positive transfection. D: Forced IFN-γR expression inhibited the radio-sensitivity of CNE-1 cells.
Discussion
Overexpression of IFN-γR has been observed in various types of human tumors, and elevated expression of IFN-γR indicated poor prognoses for patients, such as gastric carcinoma, prostate cancer, oral squamous cell carcinoma and non-small cell lung cancer. Particularly, IFN-γR hyperexpression was found to be associated with poor prognosis and reduced p27 expression in gastric carcinoma. Some studies have revealed that IFN-γR was amplified and upregulated in breast cancer. Moreover, its overexpression indicated worse tumor stage and lymphatic metastasis. However, there have been no reports on IFN-γR expression in NPC until now. Our results showed for the first time that IFN-γR expression increased in NPC, which significantly related with tumor stage and positive lymphatic metastasis. Coincidently, other studies have demonstrated that elevated IFN-γR level was correlated with poor prognosis in colorectal carcinoma [9,10]. However, no significance was shown between IFN-γR level and overall survival of NPC patients in the present study. We could not help putting forward the speculation whether IFN-γR expression affects radiotherapy outcome. Survival analysis unveiled that the overall survival rate of patients received radiation therapy was indeed affected by IFN-γR expression. Histological detection also showed a strong association betweeen IFN-γR hyperexpression and T-stage and lymphatic metastasis. Besides, in vitro assay further confirmed this point. This was the first report about the correlation between IFN-γR expression and radiation resistant of carcinoma.
Some studies reported that downregulation of IFN-γR suppressed prostate cancer cells proliferation, anchorage-independent growth and migration 11,12], while knockdown of Cks2 induced apoptosis [13,14]. Decreased IFN-γR promoted G2/M phase arrest and programmed cell death in human lung cancer cells [15]. Some study also found that overexpressin of IFN-γR in breast cancer cells repressed cell apoptosis through the MEK-Erk pathway [16]. However, no reports have described the apoptotic effects of IFN-γR expression in radiation-treated cells. In our next study, we may find out that overexpression of IFN-γR could induce apoptosis in CNE-1 cells after radiation.
To conclude, this study identified that overexpression of IFN-γR correlated with the poor prognosis of NPC patients receiving radiotherapy and elevated IFN-γR expression promoted the radiation resistance ability of NPC cells.
Conflict of interest: None declared.
Source of funding: None.
Consent: Written informed consent was obtained from the patients.
Acknowledgments: The Authors thank all the people involved in the project.
References
- Chua ML, Wee JT, Hui EP, Chan AT (2016) Nasopharyngeal carcinoma. Lancet 387: 1012-1024. [Crossref]
- Qu S, Liang ZG, Zhu XD (2015) Advances and challenges in intensity-modulated radiotherapy for nasopharyngeal carcinoma. Asian Pacific journal of cancer prevention: APJCP16:1687-1692.
- Tang LL, Chen L, Mao YP, Li WF, et al. (2015) Comparison of the treatment outcomes of intensity-modulated radiotherapy and two-dimensional conventional radiotherapy in nasopharyngeal carcinoma patients with parapharyngeal space extension. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology116:167-173.
- Swisher JF, Feldman GM (2015) The many faces of Fcgamma RI: implications for therapeutic antibody function. Immunological reviews 268:160-174.
- Nagai Y, Tsuchiya H, Runkle EA, Young PD et al. (2015) Disabling of the erbB Pathway Followed by IFN-gamma Modifies Phenotype and Enhances Genotoxic Eradication of Breast Tumors. Cell reports12:2049-2059.
- Saad S, Dunn LB, Koetters T, Dhruva A, et al. (2014) Cytokine gene variations associated with subsyndromal depressive symptoms in patients with breast cancer. European journal of oncology nursing: The official journal of European Oncology Nursing Society18:397-404.
- Chen C, Guo L, Shi M, Hu M et al. (2012) Modulation of IFN-gamma receptor 1 expression by AP-2alpha influences IFN-gamma sensitivity of cancer cells. The American journal of pathology180:661-671.
- Zhao X, Feng X, Peng D, Liu W et al. (2016) Anticancer activities of alkaloids extracted from the Ba lotus seed in human nasopharyngeal carcinoma CNE-1 cells. Experimental and therapeutic medicine12:3113-3120.
- Wang L, Wang Y, Song Z, Chu J et al. (2015) Deficiency of interferon-gamma or its receptor promotes colorectal cancer development. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research35:273-280.
- Ni C, Wu P, Zhu X, Ye J et al. (2013) IFN-gamma selectively exerts pro-apoptotic effects on tumor-initiating label-retaining colon cancer cells. Cancer letters 336:174-184.
- Wang X, Breeze A, Kulka M. (2015) N-3 polyunsaturated fatty acids inhibit IFN-gamma-induced IL-18 binding protein production by prostate cancer cells. Cancer Immunol Immunother 64:249-258.
- Wee ZN, Li Z, Lee PL, Lee ST et al. (2014) EZH2-mediated inactivation of IFN-gamma-JAK-STAT1 signaling is an effective therapeutic target in MYC-driven prostate cancer. Cell reports 8:204-216.
- Lan Y, Zhang Y, Wang J, Lin C et al. (2008) Aberrant expression of Cks1 and Cks2 contributes to prostate tumorigenesis by promoting proliferation and inhibiting programmed cell death. International journal of cancer 123:543-551.
- You H, Lin H, Zhang Z (2015) CKS2 in human cancers: Clinical roles and current perspectives (Review). Molecular and clinical oncology 3:459-463.
- Yang D, Stewart TJ, Smith KK, Georgi D et al. (2008) Downregulation of IFN-gammaR in association with loss of Fas function is linked to tumor progression. International journal of cancer122:350-362.
- Chatterji U, Riby JE, Taniguchi T, Bjeldanes EL et al. (2004) Indole-3-carbinol stimulates transcription of the interferon gamma receptor 1 gene and augments interferon responsiveness in human breast cancer cells. Carcinogenesis 25:1119-1128.