Abstract
Polycystic Ovary Syndrome (PCOS) is a common endocrine disorder that affects women in reproductive age and associates variable degrees of hyperandrogenism, anovulation and/or Polycystic Ovary Morphology (PCOM), generating different phenotypes. Due to its heterogenic etiology, there is no general treatment, but rather an individual approach for each case.
The aim of this study is to detect possible variations in clinical and biochemical outcome in PCOS cases, after treatment with combined oral contraceptive pills, metformin or inositols.
This prospective study presents 56 patients in fertile age (18–36 years old), diagnosed with PCOS, which received treatment with oral contraceptives (30mg Etinylestradiol+Dienogest), metformin (2x500mg) or inositols (2g Myoinositol). Patients were evaluated clinically and biochemically at baseline, at 3 and 6 months.
In patients treated with contraceptive pills, LH levels decreased by 77.71% after 3 months, and LH:FSH ratio with 54.01% (p=0.0005) after 6 months. There was a 52% improvement in PCOM at ultrasound examination from 3 months treatment on (p<0.0001), but no significant decrease in menstrual cycle length. Metformin proved superior in decreasing abdominal circumference and HbA1c. The inositol group had the most significant improvement after 3 months, all parameters being significantly improved apart from Ferriman-Gallwey score; menstrual cycle pattern improved significantly after 3 months of treatment (54.7%, p=0.0008).
There were significant differences in outcomes for clinical and biochemical parameters between the different treatments, yet none of them turned out superior in all main signs and symptoms, (hyperandrogenism, ovarian dysfunction and polycystic morphology). Treatment in PCOS patients should be individualised to patient’s symptoms and needs.
Keywords
Hyperandrogenism, PCOS phenotypes, Inositol, Metformin, Contraceptives
Introduction
Although polycystic ovary syndrome (PCOS) represents one of the most common endocrine disorders, affecting about one in ten women in fertile age (8-13%), it is still under diagnosed and its mechanisms not completely understood. Implications are wide and include metabolic, reproductive and psychological components. Infertility, insulin resistance and increased cardiovascular risk are the main concerns when managing these patients [1-3].
PCOS is mostly an exclusion diagnosis. Conditions like thyroid disorders, 21-hydroxylase deficiency, hyperprolactinemia, Cushing’s syndrome, hyper androgenic tumors (ovarian or adrenal), ovarian stromal hyperplasia, ovaries with physiological follicles, or the use of virilizing drugs need to be ruled out [4-6].
The confirmation of PCOS diagnosis is still made using the Rotterdam criteria and must include minimum two out of the three criteria: oligomenorrhea / Ovulatory Dysfunction (OD), clinical or biochemical evidence of hyperandrogenism (HA) and Polycystic Ovary Morphology (PCOM). In accordance with the variation of symptoms, four different phenotypes have been described. Phenotype A comprises all three elements for diagnosis (HA, OD and PCOM), phenotype B includes HA and OD, phenotype C – HA and PCOM and phenotype D, with OD and PCOM, but not HA. The first two are considered ”classic forms” of PCOS and are more frequently associated (up to 85%) with insulin resistance and variable metabolic alterations, as opposed to phenotype D (”non-HA”), where metabolic impairment was found in fewer patients, being at a lower risk to develop diabetes [1,3,7,8].
When diagnosing a new case of PCOS, phenotype assessment is required, being highly suggestive for future possible complications and more importantly for choosing the most appropriate and individualized therapeutic approach. Treatment in PCOS is long-term and may need adjustments in evolution. Treatment should target weight loss, ameliorate hormonal and reproductive disturbances and prevent comorbidities [9].
Patients should be counseled to make diet and lifestyle changes, as PCOS usually progresses with weight gain, which can precipitate development of comorbidities. Also, physical activity has been found to improve fertility and live births in women with reproductive problems [10].
Pharmacological intervention is needed in most cases, its use in PCOS is off-label but it is evidence-based. Oral Contraceptive Pills (OCPs) are recommended for women with HA and oligomenorrhea who do not target pregnancy, with favorable outcomes on hirsutism, acne, alopecia, menstrual cycle length and decreasing chance of developing endometrial hyperplasia. For achieving fertility, first-line treatment is Letrozole, with a lower risk of multiple pregnancies, but clomiphene citrate and/or metformin can be considered. Metformin is an insulin sensitizer used with success in patients with metabolic disease; it has been proved to reduce HA, normalize menstrual cycle and restore ovulation. Inositols, represented by Myo-Inositol (MI) and D-chiro-Inositol (DCI) are molecules that act as insulin messengers. Correcting a possible deficiency may improve HA, ovulatory and metabolic aspects of PCOS, but there is not enough evidence of their benefits [1,6,11-13].
The aim of the present study was to detect possible variations in clinical and biochemical outcomes in PCOS cases, after treatment with combined oral contraceptives (30mg Etinylestradiol + Dienogest), metformin (2 × 500mg) or inositols (2g Myo-Inositol). Clinical and biochemical parameters were compared at baseline, at 3 and 6 months follow up and a correlation was studied for each treatment choice.
Materials and Methods
Seventy-four women in fertile age (between 18 – 36 years old), diagnosed with PCOS, were included in the study starting with January 2017. The patients were recruited from Dr. D Medical Center in Timisoara, Romania.
Inclusion Criteria. Patients with a clear diagnosis of PCOS were included in the study (minimum 2 out of 3 Rotterdam criteria: HA, OA, PCOM), who agreed to evaluation and stayed compliant to the given treatment for the following 6 months (metformin, inositols or OCPs); other disorders causing hyperandrogenism (ovarian or pituitary tumors, Cushing’s disease, iatrogenic causes) have been excluded.
Each participant signed a written informed consent and the ethics committee approved the study of the Medical Center.
All subjects were evaluated clinically, biochemically and with imaging techniques.
Initial hormonal evaluation included Luteinizing Hormone (LH), Follicle Stimulating Hormone (FSH), LH: FSH ratio, free testosterone, Dehydroepiandrosterone sulfate (DHEA-S), prolactin (PRL), cortisol and hemoglobin A1c(HbA1c).
Clinical examinaton was performed at baseline, 3 and 6 months, including anthropometric measurements: height, weight, body mass index (BMI) and abdominal circumference. Hirsutism was evaluated using the Modified Ferriman-Gallwey score; a value ≥ 8 was assessed as hirsutism [14].
The maximum time of amenorrhea was assessed as a measure of OD at baseline. In the follow-up examination at 3 and 6 months, the maximum time of amenorrhea was exchanged by menstrual cycle length, as a measure of OD. When comparing maximum time of amenorrhea with menstrual cycle length, the number of months was multiplied by 28 to yield the consecutive time in days.
In order to determine presence of PCOM, transvaginal ultrasound of the ovaries was performed. Positive for PCOM were considered cases with more than twelve follicles or an ovarian volume greater than 10 ml [1].
A prospective evaluation of clinical parameters was made: BMI, Ferriman-Gallwey (FG) score, menstrual cycle’s assessment. Hormonal and metabolic parameters were re-evaluated after 6 months of treatment, as well as ultrasound appearance.
The Therapeutic Intervention. The therapeutic intervention included three treatment choices and the study group was subdivided in 3 groups, according to their treatment option: 24 patients in study group 1 were treated with inositols (2g Myo-Inositol). 25 patients in study group 2 were treated with OCPs (30mg Etinylestradiol + Dienogest), and 25 patients in study group 3 were treated with metformin (Metformin 2 x 500mg).
Statistical Analysis was carried out using SPSS and a threshold value for probability (p) < 0.05 was considered statistically significant in order to reject the null hypothesis. The data was expressed as mean values and standard deviations. The clinical and biochemical characteristics were compared to each other between the study groups using the unpaired t-test; the paired t-test was applied for different parameters comparison at baseline and post-treatment. Multifactorial analysis of variance (ANOVA) test was used univariate to compare the three study group parameters at baseline, then it was applied multivariate to compare outcomes for all study groups and to detect differences in parameter values at baseline, 3 and 6 moths of follow-up.[15]
Results
The entire study group included 74 patients with a mean age of 26.91 ± 3.92, between 18 and 36 years old. The cases were subdivided in 3 categories as follows: study group 1 represents the patients that received treatment with Inositol and includes 24 patients, study group 2 is made up of 25 patients treated with OCPs and study group 3 includes 25 patients that received treatment with Metformin.
The evaluated characteristics for the three study groups at baseline are presented in Table 1 as mean value ± standard deviation as well as the comparison of parameters at baseline between all three study groups using the monofactorial ANOVA test.
Table 1. Clinical and biochemical characteristics at baseline and value comparison using monofactorial ANOVA test for all studied parameters between study groups 1–3
|
Parameters |
Group 1 |
Group 2 |
Group 3 |
F value |
p value |
Critical f value |
|
BMI (kg/m²) |
31.35 |
25.71 |
31.70 |
10.77 |
0.0001 |
3.12 |
|
FG score |
17.75 |
16.84 |
12.12 |
28.92 |
< 0.0001 |
3.12 |
|
Max amenorrhea (months) |
3.38 |
1.24 |
3.26 |
11.55 |
< 0.0001 |
3.12 |
|
Abdominal circumference (cm) |
100.00 |
81.04 |
99.92 |
2.44 |
0.094 |
3.12 |
|
Free testosterone (mIU/ml) |
0.02 |
0.02 |
0.0 |
7.72 |
0.001 |
3.12 |
|
DHEAS (mg/dl) |
413.54 |
411.44 |
337.24 |
6.52 |
0.003 |
3.12 |
|
PRL (mIU/ml) |
591.46 |
550.44 |
374.12 |
11.87 |
< 0.0001 |
3.12 |
|
LH (mIU/ml) |
13.00 |
13.996 |
14.13 |
0.68 |
0.508 |
3.12 |
|
FSH (mIU/ml) |
5.27 |
6.16 |
5.54 |
1.16 |
0.323 |
3.20 |
|
LH:FSH ratio |
2.50 |
2.37 |
2.64 |
0.79 |
0.459 |
3.12 |
|
Cortisol (mg/dl) |
18.77 |
17.15 |
21.75 |
5.90 |
0.004 |
3.12 |
|
HbA1c (%) |
6.02 |
5.85 |
6.16 |
1.49 |
0.233 |
3.12 |
A significant difference (p-value <0.05) was found in BMI (p=0.0001), FG score (p<0.0001), maximum amenorrhea (p<0.0001), testosterone level (p=0.001), DHEA-S level (p=0.003), prolactin (PRL) (p<0.0001) and cortisol levels (p=0.004).
Figure 1 presents the mean value of the studied parameters in group 1 (24 patients) at baseline and the evolution of parameters (mean values) after they started treatment with inositol, at 3 and 6 months follow-up.
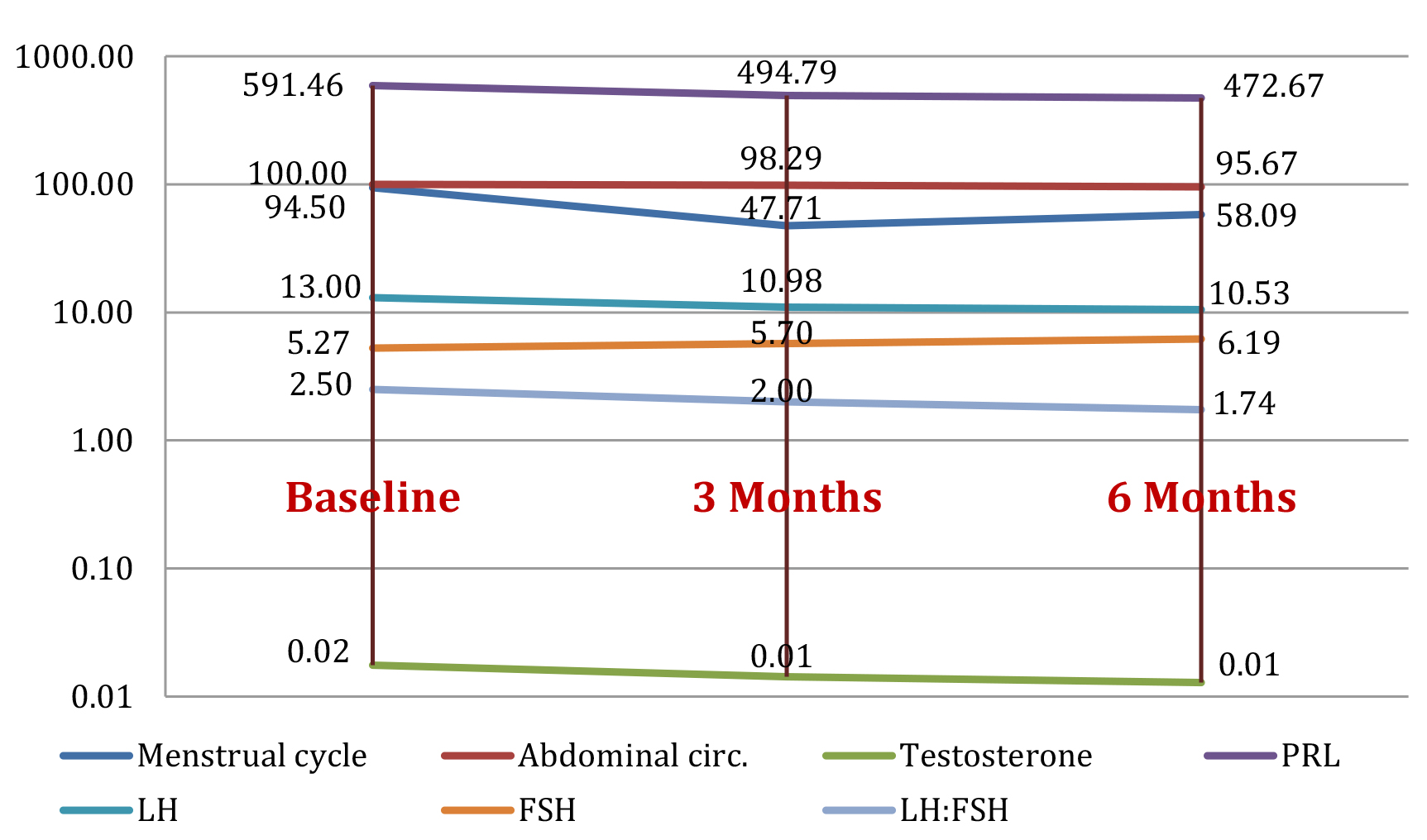
Figure 1. Study group 1: evolution of parameters (mean value) at baseline, and at 3 months and 6 months after initiating treatment with inositols
Figure 2 shows evolution for the mean value of the parameters for the 25 cases in group 2 initially and as tested parameters change after being treated with OCPs at3 and 6 months follow-up.
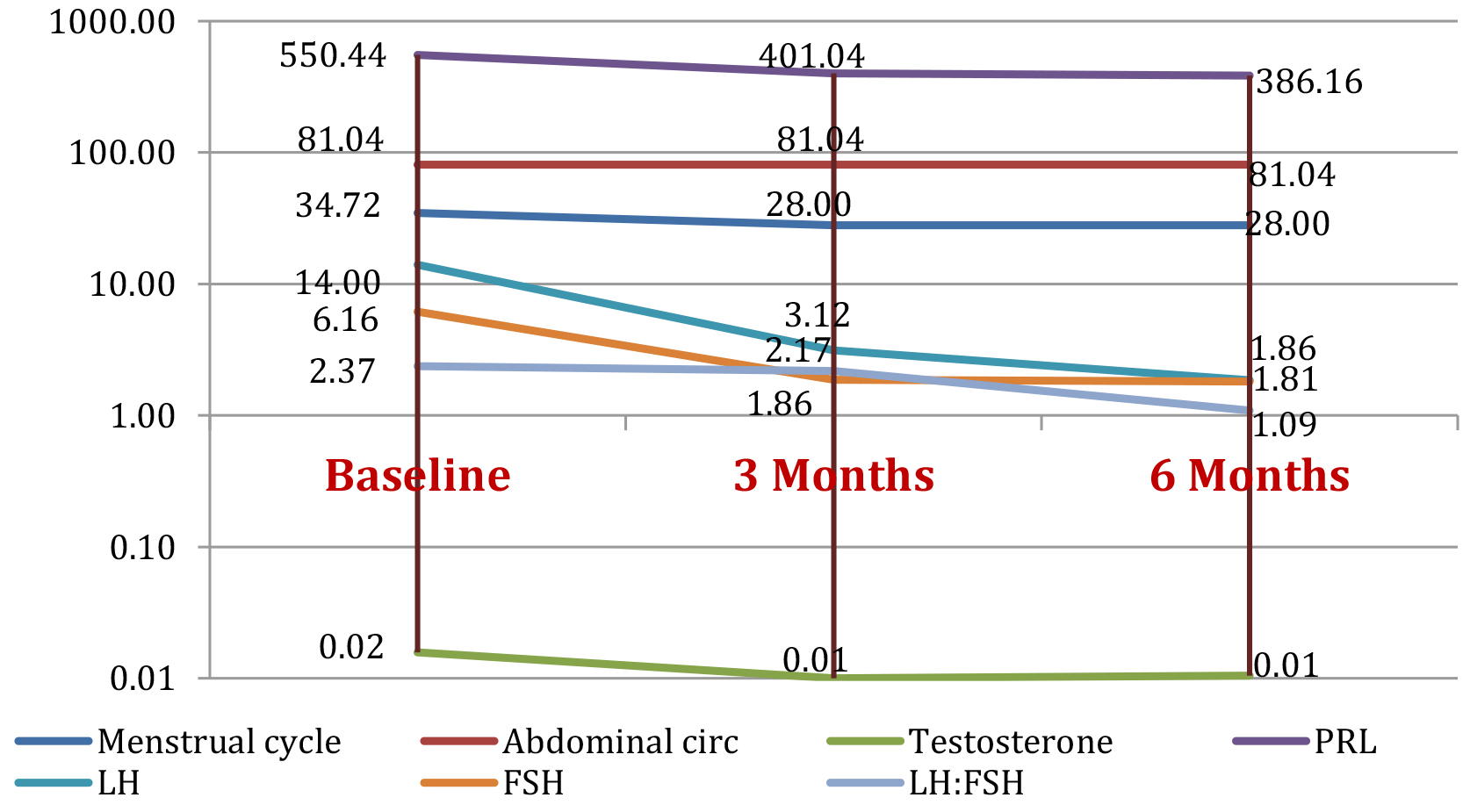
Figure 2. Study group 2: evolution of parameters (mean value) at baseline, and at 3 months and 6 months after initiating OCP treatment
Figure 3 presents the summarized means of the parameters in group 3 after the 25 patients entered the program and were treated with metformin, changes in their testing parameters could be observed at respectively 3 and 6 months follow up.
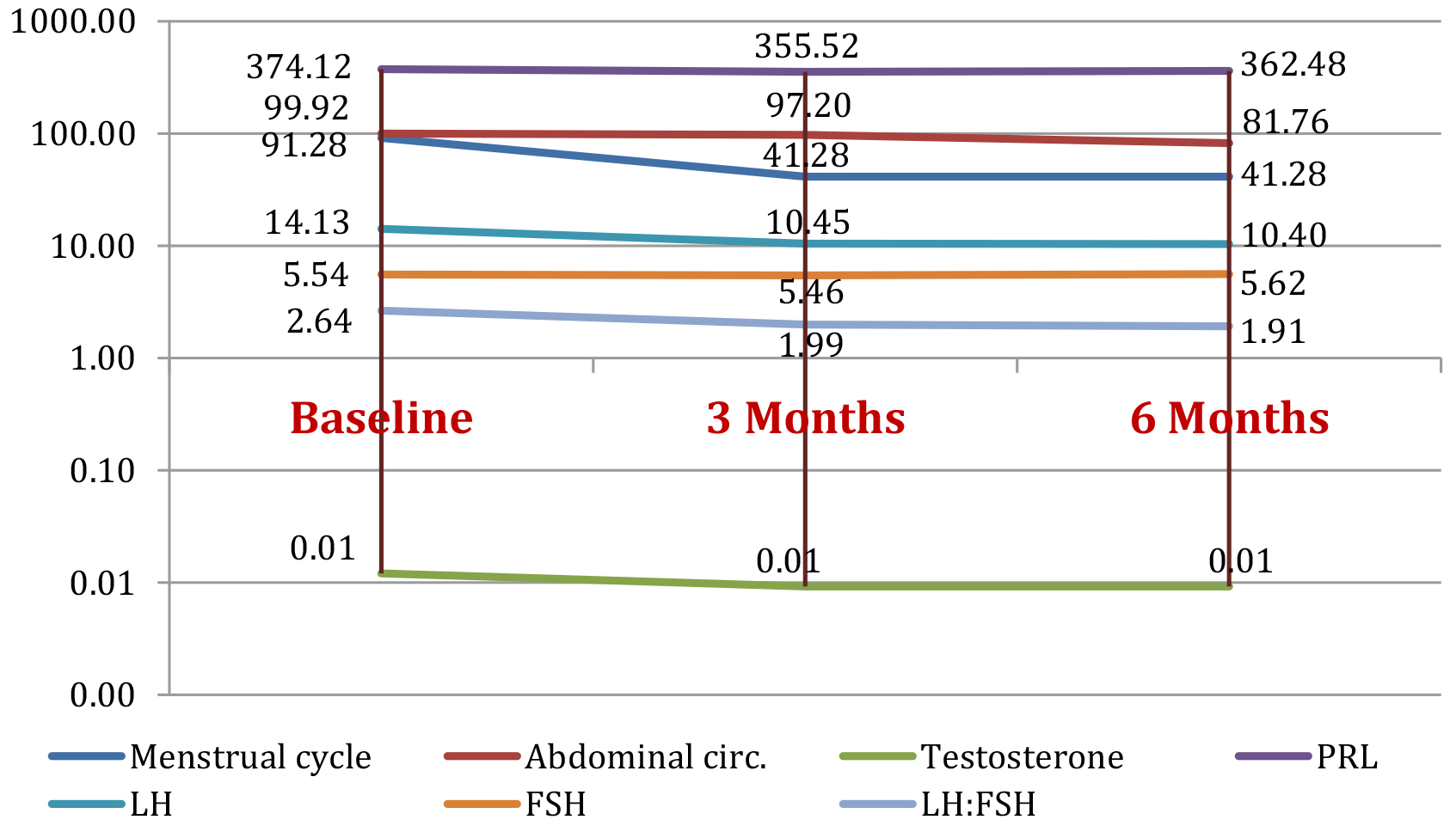
Figure 3. Study group 3: parameters evolution (mean value) at baseline and at 3 months and 6 months after metformin treatment
Data in Table 2 shows the presence of PCOM on ultrasound evaluation in study groups 1-3 at baseline and in evolution after starting each type of treatment respectively, at 3 and 6 months follow-up. For group 1, all 24 patients (100%) were positive at baseline examination, after 3 months the positive cases were reduced to 16 and 8 negatives and continued to decrease to 12 positives (50%) and 12 negatives at 6 months follow-up. For group 2, only 12 patients (48%) out of the total 25 were positive for PCOM at initial exam, and all of them became negative at 3 and 6 months after OCP treatment. In group 3, nineteen patients (76%) were PCOM positive at baseline, after 3 months of this reduced to 13 positives (52%) and 12 negatives and decreased further to 9 positives (36%) and 16 negatives at 6 months follow up.
Table 2. Ultrasound PCOM evaluation at baseline, 3 months and 6 months follow up in study groups 1-3
|
Parameter |
Group 1 (inositol) |
Group 2 (OCP) |
Group 3 (metformin) |
|||||||
|
Evaluation |
B |
3m |
6m |
B |
3m |
6m |
B |
3m |
6m |
|
|
PCOM |
Positive |
24 |
16 |
12 |
12 |
0 |
0 |
19 |
13 |
9 |
|
100% |
66.6% |
50% |
48% |
0% |
0% |
76% |
52% |
36% |
||
|
Negative |
0 |
8 |
12 |
13 |
25 |
25 |
6 |
12 |
16 |
|
|
0% |
33.4% |
50% |
52% |
100% |
100% |
24% |
48% |
64% |
||
In Table 3 the patient parameters are compared at baseline and 3 months of treatment in all three-study groups using the paired T-test. A cut-off for the p-value of 0.05 was considered as statistically significant. For study group 1, treated with inositol there was no change in FG score values. Statistically significant differences were seen in menstrual cycle length (p=0.006), abdominal circumference (p=0.01), testosterone levels (p<0.0001), PRL levels (p=0.011), LH levels (p=0.0002), FSH levels (p=0.013), LH:FSH ratio (p=0.001), HbA1c levels (p<0.0001) and at PCOM ultrasound evaluation (p=0.003). In group 2 that received OCPs as therapy for three months, significant differences have been detected for menstrual cycle length, PRL, LH, FSH levels and PCOM and for the metformin-treated group 3 statistically significant differences were found in menstrual cycle length, abdominal circumference, testosterone, LH levels and LH:FSH ratio.
Table 3. Statistical analysis: T-Test initial vs 3 months in study groups 1,2 and 3
|
Parameters |
Group 1 |
Group 2 |
Group 3 |
|||
|
t score |
p value |
t score |
p value |
t score |
p value |
|
|
Menstrual cycle |
3.00 |
0.006 |
8.22 |
< 0.0001 |
3.81 |
0.0008 |
|
Abdominal circ |
2.82 |
0.010 |
0.93 |
0.359 |
5.35 |
< 0.0001 |
|
Testosterone |
5.91 |
< 0.0001 |
0.90 |
0.377 |
3.89 |
0.0007 |
|
PRL |
2.75 |
0.011 |
5.00 |
< 0.0001 |
0.41 |
0.6836 |
|
LH |
4.49 |
0.0002 |
9.53 |
< 0.0001 |
4.66 |
0.0001 |
|
FSH |
-2.68 |
0.013 |
10.35 |
< 0.0001 |
0.23 |
0.8197 |
|
LH:FSH |
3.84 |
0.001 |
0.28 |
0.783 |
4.05 |
0.0005 |
|
HbA1c |
7.13 |
< 0.0001 |
-0.10 |
0.918 |
6.76 |
< 0.0001 |
|
PCOM |
3.39 |
0.003 |
4.71 |
0.0001 |
1.6 |
0.1102 |
When comparing baseline group characteristics with the ones at 6 months of treatment (see Table 4 below), the improvement continued for all categories. In the inositol–group 1, statistically significant values were attained for all evaluated parameters. In the 2nd group, treated with OCPs, significant improvement was found in menstrual cycle (p<0.0001), testosterone levels (0.047), PRL, FSH, LH levels and LH:FSH ratio, A1c and PCOM (p<0.0001) Abdominal circumference was also lower, but not significantly. For group 3, that received metformin treatment, statistically significant differences were seen in menstrual cycle length (p=0.0008), abdominal circumference (p<0.0001), testosterone levels (p=0.0003), LH levels (p=0.0001), LH:FSH (p=0.0002) and at ultrasound (p=0.0049).
Table 4. Statistical analysis: T-Test baseline vs 6 months of treatment for study groups 1,2 and 3
|
Parameters |
Group 1 |
Group 2 |
Group 3 |
|||
|
t score |
p value |
t score |
p value |
t score |
p value |
|
|
Menstrual cycle |
3.00 |
0.006 |
8.88 |
< 0.0001 |
3.81 |
0.0008 |
|
Abdominal circ |
5.77 |
< 0.0001 |
0.93 |
0.359 |
6.79 |
< 0.0001 |
|
Testosterone |
8.09 |
< 0.0001 |
2.09 |
0.047 |
4.28 |
0.0003 |
|
PRL |
3.24 |
0.004 |
6.00 |
< 0.0001 |
0.26 |
0.7993 |
|
LH |
4.97 |
0.0001 |
14.92 |
< 0.0001 |
4.52 |
0.0001 |
|
FSH |
-3.75 |
0.001 |
11.63 |
< 0.0001 |
-0.25 |
0.8071 |
|
LH:FSH |
5.07 |
< 0.0001 |
7.18 |
< 0.0001 |
4.49 |
0.0002 |
|
HbA1c |
4.80 |
0.0001 |
4.71 |
0.0001 |
3.10 |
0.0049 |
|
PCOM |
3.00 |
0.006 |
8.88 |
< 0.0001 |
3.81 |
0.0008 |
Table 5 presents the difference between the three study groups for each parameter at baseline and 6 months, using the MANOVA test.
Table 5. MANOVA test: baseline vs 6 months – differences between groups
|
Parameters |
F value |
p value |
Critical f value |
|
Menstrual cycle |
19.68 |
< 0.0001 |
3,06 |
|
Abdominal circ. |
4.38 |
0.014 |
3,06 |
|
Testosterone |
7,24 |
0,001 |
3,06 |
|
PRL |
19,54 |
<0,0001 |
3,06 |
|
LH |
30,53 |
<0,0001 |
3,06 |
|
FSH |
34,85 |
<0,0001 |
3,06 |
|
LH:FSH |
9,96 |
0,0001 |
3,06 |
|
eco PCOS |
21,20 |
<0,0001 |
3,06 |
The data was compared initially and at 3 months of therapy for the study groups two by two, as shown in Table 6. When the inositol and OCP groups are compared, statistically significant differences are found for FG score, menstrual cycles, abdominal circumference, PRL, LH, FSH and ultrasound polycystic morphology. When groups 1 and 3 were compared, differences were only noticed for testosterone, PRL and menstrual cycles length and as for comparing groups 2 and 3 significant for FG score, menstrual cycles, abdominal circumference, LH, FSH and PCOM.
Table 6. T-Test baseline vs 3 months – study groups comparison
|
Parameters |
group 1 vs group 2 |
group 1 vs group 3 |
group 2 vs group 3 |
|||
|
t test |
p value |
t test |
p value |
t test |
p value |
|
|
FG score |
5.40 |
< 0.0001 |
0.79 |
0.431 |
2.77 |
0.008 |
|
Menstrual cycle length |
3.28 |
0.003 |
7.26 |
< 0.0001 |
-2.45 |
0.022 |
|
Abdominal circ. |
4.89 |
< 0.0001 |
0.32 |
0.751 |
-4.62 |
< 0.0001 |
|
Testosterone |
0.43 |
0.674 |
5.07 |
< 0.0001 |
1.12 |
0.275 |
|
PRL |
3.33 |
0.002 |
4.74 |
< 0.0001 |
1.34 |
0.185 |
|
LH |
7.90 |
< 0.0001 |
0.67 |
0.505 |
-7.72 |
< 0.0001 |
|
FSH |
13.92 |
< 0.0001 |
0.63 |
0.530 |
-10.82 |
< 0.0001 |
|
LH:FSH |
-0.24 |
0.809 |
0.07 |
0.948 |
0.26 |
0.796 |
|
HbA1c |
-0.43 |
0.671 |
-0.42 |
0.679 |
-0.02 |
0.981 |
|
PCOM |
6.78 |
< 0.0001 |
1.03 |
0.306 |
-5.10 |
< 0.0001 |
Groups were compared again after 6 months of treatment using the unpaired t-test. Results are shown in Table 7.
Table 7. T-Test baseline vs 6 months – study groups comparison
|
Parameters |
group 1 vs group 2 |
group 1 vs group 3 |
group 2 vs group 3 |
|||
|
t test |
p value |
t test |
p value |
t test |
p value |
|
|
Menstrual cycle length |
6.84 |
< 0.0001 |
2.45 |
0.018 |
-2.45 |
0.022 |
|
Abdominal circ. |
4.32 |
0.0001 |
4,08 |
0,0002 |
-0.20 |
0.843 |
|
Testosterone |
0,93 |
0,361 |
5,13 |
<0,0001 |
0.77 |
0.445 |
|
PRL |
4,56 |
0,0000 |
4,39 |
0,0001 |
0.92 |
0.363 |
|
LH |
16,91 |
0,0000 |
0,18 |
0,856 |
-15.60 |
< 0.0001 |
|
FSH |
16,51 |
0,0000 |
1,71 |
0,094 |
-14.85 |
< 0.0001 |
|
LH:FSH |
5,48 |
0,0000 |
-1,22 |
0,230 |
-5.59 |
< 0.0001 |
|
PCOM |
4,80 |
0,0001 |
0,98 |
0,333 |
-3.67 |
0.001 |
Discussion
A number of studies attempted to compare different treatment approaches for women with PCOS and data is still inconclusive, outcomes depending individually. While metformin and OCPs have been extensively studied, literature data on inositol therapy is still poor and needs further study. The present study aimed to compare inositol with the more commonly used metformin and oral contraceptives and determine whether one of them is superior to others. No real differences were found when comparing the results of the different forms of T-Test with the results of ANOVA and MANOVA tests.
As for OCPs, normal menstrual cycles were restored at 3-month follow-up in 100% of cases, but the decrease in menstrual cycle length was not significant (19.35%, p=0.359). Improvement in ultrasound aspect of the ovaries was found in all patients as soon as 3 months of treatment (p<0.0001). FG score was also significantly improved after 6 months of treatment and PRL status at 6 months after initiation of OCPs (29,85%, p<0.0001). Our findings show a decrease in testosterone levels by 50%, in LH levels by 77,71% after 3 months and of 69.81% (p<0.0001) in FSH levels from 3 month follow up on. LH to FSH ratio decreased consecutively by 54.01% (p=0.0005) after 6 months. This confirms existing data on improving testosterone levels and LH-FSH profile. OCPs have been also found to normalize free androgen index and SHBG values. Abdominal circumference did not seem to improve in the OCP group, but this could be explained by the baseline value close to the normal upper limit for the studied group. There is also literature data showing a negative impact of OCPs on BMI. Adding metformin to therapy was proposed in order to attenuate this effect [1,16,17].
Regarding metformin therapy, its role in reducing symptoms of PCOS is supported by literature, with the exception of hirsutism, although in some studies it was proven to reduce acne, hirsutism and improve fertility. Menstrual cycle pattern normalized significantly by 54,78% at 3 months follow-up (p=0.0008) and remained stable at 6 months. There was a clear improvement in free testosterone after treatment with metformin starting from 3 months follow-up (p=0.0007) and continuing to decrease and a decrease of LH:FSH ratio by 27.65% (p=0.0002) for patients treated with metformin for 6 months. Other studies support our data with similar results. One study described a decrease in ovarian volume by 10% (p=0.001) marking an improvement at ultrasound examination, which was also found in our study after 6 months (47,37%, p=0.0049). Metabolic parameters were, as expected, improved with metformin therapy in the majority of studies. A decrease by 4.81 % in HbA1c after only 3 months of treatment (p<0.0001) was noticed, while literature data shows differences of up to 15% after 6 months. Abdominal circumference decreased by 8.17% (p<0.0001) after the same time period [7,18-25].
More research papers on use of inositols in women with PCOS have been published for the past couple of years. Our paper aimed to bring a contribution to current knowledge. The most significant improvement in all our study groups was assessed for the inositol group at 3-month follow-up. All the monitored parameters, both endocrine and metabolic, have been significantly improved with the exception of FG score. Other publications have found an improvement with inositol therapy even for this parameter. A decrease of 38,53% (p=0.006) in menstrual cycle length was found, which is extremely comparative other literature findings, but mean length value is still greater than normal. PRL levels decreased by 20,08% (p=0.004) after 6 months treatment, LH levels recorded a 15,54% decrease (p=0.0002) after 3 months and FSH up to 17.46% (p=0.001) after 6 months of treatment; we did not find a significant improvement for FSH values in other publications. LH:FSH ratio decreased by 20% (p=0.001) after 3 months of treatment, which was similar to other findings. Testosterone levels improved by 50%. A decrease by up to 4.33% (p<0.0001) was documented for abdominal circumference, also for HbA1c a significant 3.82% decrease was found (p=0.010). Our work supports other papers findings, a decrease in BMI and in HbA1c have also been reported. As to ultrasound examination, we found a statistically significant improvement of 25% (p=0.003, p=0.0001) regarding PCOM with each 3 months, yet to our knowledge other studies could not find a significant improvement [26-29].
OCPs vs Metformin
The present study showed favorable outcomes for OCPs after 3 months of treatment, a statistically significant difference in effectiveness was in favor of OCP treatment in regard to decreasing FG score and LH levels. When evaluating of PCOM aspect evolution, OCPs are also superior (all patients were negative after 3 months), but there was a significant difference in initial parameters which favored the OCP group.Metforminhad better results in decreasing abdominal circumference and menstrual cycle length, but it should be taken into account that the values for the OCP group were closer to normal at baseline and this could benefit the metformin group to achieve a greater improvement. Regarding HbA1c, metformin was also superior compared to OCP, which did not attain a significant improvement.
Multiple studies compared efficacy of these two agents, most of the result were comparable to ours. Metformin has been shown to improve most metabolic parameters, but also OD and fertility in some cases, while OCPs had better outcomes in ameliorating HA symptoms and endocrine parameters [1,22].
Metformin vs Inositol
Combining the stastical results after 3 month follow-up, inositols clearly showed to be superior in decreasing abdominal circumference, improving PRL levels, and twice as effective regarding PCOM aspect. There was a statistically significant difference in effectiveness in favor of metformin for menstrual cycle length; for HbA1c, metformin was superior but not statistically significant.
There is only few literature data in this regard and results are conflicting. Some recent studies found improvements in the endocrine and clinical outcomes in clear favor to metformin while other report the two are equally effective in normalizing both metabolic and clinical characteristics. Given the lack of conclusive data, current guidelines do not support use of inositols in treating women with PCOS and considers its use still experimental.[1,26,27,29]
Inositol vs OCPs
Currently there is no data comparing inositols and oral contraceptive outcomes. Our findings show superior results for OCPs on endocrine parameters: PRL, LH and LH:FSH. OCPs were also quicker in improving PCOM (100% negative for PCOM at 3 months). Worth mentioning that initial values have already been started in favor of the OCP group at baseline. The inositol group took at least twice as long reach the same improvement (%). Similarly to metformin, the insulin sensitizer was better than OCPs in improving abdominal circumference and menstrual cycle length.
The multiple initial differences between the study groups, the relatively short follow-up of only 3 months for HbA1c and FG score, the different time from diagnosis and the lack in monitoring lifestyle intervention are some of the limitations of this study. One of its strengths was comparing OCP treatment with inositol, which to our knowledge was not done to this extent.
Conclusion
The findings of this study showed superior outcomes for OCPs in improving endocrine parameters: PRL, LH, FSH, LH:FSH ratio and polycystic morphology aspect on ultrasound. Inositols and metformin also improved these parameters, but to a lower extent.
Both insulin sensitizers (metformin and inositol) turned out triumphant in reducing abdominal circumference and menstrual cycle length, with better results for metformin in restoring menstrual cycle pattern, while an exceptional performance was described for the inositol group in improving abdominal circumference, testosterone and PRL levels.
Some of the results may have been influenced by a distribution closer to the normal range that could benefit certain therapy outcomes. A randomization at baseline might be of help in future studies.
Given the little data and the big potential of inositol treatment, there is a real need for more extensive research in this regard. Up until now, studies showed great outcomes for inositols, and considering the absence of the typical gastrointestinal side effects of metformin, they may be considered a real alternative to metformin or as complementary therapy to OCPs.
Significant differences in clinical and biochemical parameters have been found between outcomes for the diverse treatment choices, still none of them turned out superior in ameliorating all main components (HA, OD and PCOM). The treatment in each case of PCOS should be individualized to the patient’s symptoms and needs.
Abbreviations
PCOS – Polycystic ovary syndrome
MI – Myo-Inositol
DCI – D-chiro-Inositol
OCP – Oral contraceptive pills
PCOM – Polycystic ovarian morphology
FG score – Ferriman-Gallwey score
HA – Hyperandrogenism
OD – Ovarian dysfunction
MANOVA – Multifactorial analysis of variance
References
- Teede H, Misso M, Costello M, Dokras A, Laven J, et al (2018) International evidence-based guideline for the assessment and management of polycystic ovary syndrome. National Health and Medical Research Council (NHMRC) 2018.
- Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, et al (2015) American Association Of Clinical Endocrinologists, American College Of Endocrinology, And Androgen Excess And Pcos Society Disease State Clinical Review: Guide To The Best Practices In The Evaluation And Treatment Of Polycystic Ovary Syndrome – Part 1. Endocr Pract.
- Azziz R (2016) Introduction: Determinants of polycystic ovary syndrome. Fertil Steril.
- Karkanaki A, Vosnakis C, Panidis D (2011). The clinical significance of anti-müllerian hormone evaluation in gynecological endocrinology. Hormones.
- Meier RK (2018) Polycystic Ovary Syndrome. Nursing Clinics of North America.
- S.m S, K.a P (2014). Epidemiology, diagnosis, and management of polycystic ovary syndrome Clin Epidemiol.
- H.J. T, M.L. M, M.F. C, A. D, J. L, et al (2018) Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod.
- Bani Mohammad M, Majdi Seghinsara A. Polycystic Ovary Syndrome (PCOS), Diagnostic Criteria, and AMH. Asian Pac J Cancer Prev 18:17–21.
- LJ M, SK H, RJ N, HJ T, Moran Lisa J et al (2011). Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev.
- Mena GP, Mielke GI, Brown WJ (2019). The effect of physical activity on reproductive health outcomes in young women: a systematic review and meta-analysis. Hum Reprod Update.
- Jin P, Xie Y (2018). Treatment strategies for women with polycystic ovary syndrome. Gynecological Endocrinology.
- Kalra B, Kalra S, Sharma JB (2016) The inositols and polycystic ovary syndrome. Indian J Endocrinol Metab 20: 720–4.
- Bednarska S, Siejka A (2017) The pathogenesis and treatment of polycystic ovary syndrome: What’s new? Adv Clin Exp Med.
- Aswini R, Jayapalan S (2017) Modified Ferriman–Gallwey score in hirsutism and its association with metabolic syndrome. Int J Trichology.
- Kissling GE (2017) Statistical methods. In: The Clinical Chemistry of Laboratory Animals, Third Edition.
- Feng W, Jia YY, Zhang DY, Shi HR (2016). Management of polycystic ovarian syndrome with Diane-35 or Diane-35 plus metformin. Gynecol Endocrinol.
- Luque-Ramírez M, Nattero-Chávez L, Ortiz Flores AE, Escobar-Morreale HF (2018). Combined oral contraceptives and/or antiandrogens versus insulin sensitizers for polycystic ovary syndrome: A systematic review and meta-analysis. Hum Reprod Update.
- Jaganmohan C, Vannan M, Ali A, S P (2017) Evaluation of Clinical Efficacy of Metformin Therapy in Polycystic Ovary Syndrome. J Young Pharm.
- Pasquali R, Gambineri A (2013) THERAPY OF ENDOCRINE DISEASE: Treatment of hirsutism in the polycystic ovary syndrome. Eur J Endocrinol.
- Dardzińska JA, Rachoń D, Kuligowska-Jakubowska M, Aleksandrowicz-Wrona E, Płoszyński A, et al (2014) Effects of metformin or an oral contraceptive containing cyproterone acetate on serum C-reactive protein, interleukin-6 and soluble vascular cell adhesion molecule-1 concentrations in women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes.
- Cosma M, Swiglo BA, Flynn DN, Kurtz DM, LaBella ML, et al (2008). Insulin sensitizers for the treatment of hirsutism: A systematic review and metaanalyses of randomized controlled trials. Journal of Clinical Endocrinology and Metabolism.
- Morin-Papunen L, Vauhkonen I, Koivunen R, Ruokonen A, Martikainen H, et al (2003). Metformin versus ethinyl estradiol-cyproterone acetate in the treatment of nonobese women with polycystic ovary syndrome: A randomized study. J Clin Endocrinol Metab.
- Tao T, Wu P, Wang Y, Liu W (2018). Comparison of glycemic control and β-cell function in new onset T2DM patients with PCOS of metformin and saxagliptin monotherapy or combination treatment. BMC Endocr Disord.
- Celik O, Acbay O (2012) Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Invest.
- Seyam E, Hefzy E (2018). Long-term effects of combined simvastatin and metformin treatment on the clinical abnormalities and ovulation dysfunction in single young women with polycystic ovary syndrome. Gynecol Endocrinol.
- Jamilian M, Farhat P, Foroozanfard F, Afshar Ebrahimi F, Aghadavod E, et al (2017). Comparison of myo-inositol and metformin on clinical, metabolic and genetic parameters in polycystic ovary syndrome: A randomized controlled clinical trial. Clin Endocrinol (Oxf).
- Fruzzetti F, Perini D, Russo M, Bucci F, Gadducci A (2017). Comparison of two insulin sensitizers, metformin and myo-inositol, in women with polycystic ovary syndrome (PCOS). Gynecol Endocrinol.
- Artini PG, Di Berardino OM, Papini F, Genazzani AD, Simi G, et al (2013). Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecol Endocrinol.
- V. T, D. R, V. I, S. DC, C. DF, et al (2017). Metformin vs myoinositol: which is better in obese polycystic ovary syndrome patients? A randomized controlled crossover study. Clin Endocrinol (Oxf).