Abstract
Background: Head and neck cancer (HNC) patients experience significant weight loss before diagnosis, during and after treatment, and even during the first year of follow-up. However, the prognostic value of weight loss depends on body mass index, and this may be associated with low skeletal muscle mass, masking its loss. Thus, body composition changes occurring during HNC management warrant further investigation.
Objective: The aim of this scoping review was to evaluate body composition changes and the methods to assess it in HNC patients under oncological treatment with curative intent.
Inclusion Criteria: All published studies in English, Spanish and Portuguese during 2000-2019 focusing on body composition changes in HNC patients aged 18 years or older in the context of treatment with curative intent were considered. Surgical treatment approach was excluded to avoid excess heterogeneity. A three-step search strategy was undertaken.
Presentation of results: HNC patients suffer from serious loss of lean body mass, skeletal muscle or free fat mass after treatment compared with baseline. This can be demonstrated either by triceps skin fold thickness, bioelectrical impedance analysis, dual-energy x-ray absorptiometry or computed tomography. Nutritional deterioration occurs up to 8-12 months after treatment and has a remarkable impact on survival, quality of life, and risk for complications.
Conclusion: HNC patients experience a significant depletion of lean body mass, fat-free mass and skeletal muscle, accompanied by body fat mass, while undergoing (chemo) radiotherapy. Bioelectrical impedance analysis seems to be a feasible body composition assessment tool as it is inexpensive and non-invasive and usually readily available.
Keywords
Head and Neck Cancer, Body Composition, Skeletal Muscle, Lean Body Mass, Adipose Tissue, Fat Free Mass
Background
Head and neck cancer (HNC) is a term that refers to a heterogenous group of cancers that occur in the upper aerodigestive tract i.e. oral cavity, pharynx, larynx, paranasal sinuses, nasal cavity or salivary glands [1, 2]. Certain subtypes of these cancers demonstrate a strong increasing incidence and in general they are related to a low survival outcome. The most frequently found risk factors for HNC are the use of alcohol, tobacco, human papillomavirus (HPV)/Epstein-Barr virus (EBV) infection and poor oral hygiene3. Given their complexity and location, interference with the anatomical and physiological characteristics, the tumors and their treatment are able to promote aesthetic alterations and disturbance of functions such as phonation, swallowing, hearing and breathing [2, 4]. Dysphagia (difficulty in swallowing) is a prevalent risk factor for morbidity prior, during and following oncological treatment for HNCs, affecting most patients at some stage over the course of treatment, and is often rated as the most significant factor affecting quality of life amongst HNC survivors [1, 5]. Prior to treatment, HNC patients may experience swallowing dysfunction due to pain, obstruction or an uncoordinated swallowing mechanism[5], contributing to insufficient dietary intake, which may occur if the estimated energy intake is <60% of the individual requirement for more than 1 and 2 weeks [6]. However, not only the location of the tumor may result in problems in eating and drinking, but the cancer treatments (surgery and (chemo)radiotherapy either alone or in combination) also cause, in addition to dysphagia, alterations in swallowing function, which may persist for several months or even years, as xerostomia, thick saliva, difficulty in chewing, anorexia and nausea/vomiting [1-8]. These symptoms, either related to the acute toxicity or the anatomic changes caused by these treatments, may exacerbate nutrition deterioration by compromising dietary intake [2]. Even partial reduction in dietary intake (i.e. daily deficit >25%, >50%, or >75% of energy requirements) results in large caloric deficits over time, and the expected duration, as well as the degree of depletion of body reserves, should be considered. Both conditions result in weight loss and, consequently, in body mass index (BMI) reduction, which may be severe [6]. Apparently, age, race, gender, smoking and alcohol consumption, and radiation dose, do not independently predict severe weight loss [7]. The negative energy balance and skeletal muscle mass (SMM) loss observed in cancer patients is driven by a combination of reduced food intake and metabolic derangements (e.g. elevated resting metabolic rate, insulin resistance, lipolysis, and proteolysis which aggravate weight loss and are caused by systemic inflammation and catabolic factors), which may be host- or tumor-derived [6].
When compared with other cancer-patient populations, patients with HNC have the second highest prevalence of malnutrition, after upper gastrointestinal tract cancer patients [9]: 20-67% are malnourished or at high risk of becoming malnourished at diagnosis [7] and this will worsen throughout the treatment [2]. Significant weight loss (i.e., the involuntary weight loss of 5% body weight in 1 month or 10% in 6 months)[10] is a common phenomenon before HNC diagnosis, during and after treatment, and occurs for up to a year following treatment [7]. A meta-analysis conducted by Couch et al. (2015) showed a relation between the extent and prevalence of weight loss and the location and stage of the tumor. Patients with advanced-stage HNC (stage III/IV) experience weight loss significantly more often when compared with those with early-stage (stage I/II) disease [10]. Weight loss alone is often the most common clinical measurement of cachexia [10] and forms one of the independent negative prognostic factors [2] for HNC patients, having a negative impact on quality of life (QOL) and morbidity as well [10]. Cancer cachexia is a term that refers to a multifactorial syndrome defined by an ongoing loss of SMM (with or without loss of fat mass) unable to be fully reversed by conventional nutritional support and leading to progressive functional impairment [11]. An early detection of malnutrition aims to improve oncological outcomes, and minimize acute toxicities, treatment interruptions and enhance survival [2]. Body composition (BC) has gained increasing interest in oncology and refers to the amount and distribution of lean tissue and adipose tissue in the human body [12]. The published studies have shown the importance of the changes in BC in various cancer patients [2] and more specifically loss of SMM, with or without loss of fat has proven to be a significant parameter [8]. Further, muscle loss determines the limiting dose of some antineoplastic drugs due to high distribution volume in adipose tissue, resulting in a slower drug elimination [2], and in a higher chemotherapy toxicity, and increase in mortality [8]. Different parameters can be used to assess BC, but the best-known parameter is BMI [12]. However, the role of this anthropometric tool is still unclear in HNC patients [8]. In recent years, several studies have shown a clear dissociation between total body weight loss and SMM loss, reflecting the increased prevalence of obesity in the population. The prognostic value of weight loss depends on the BMI, and this may be associated with low SMM, masking its loss. Thus, weight loss itself poorly predicts outcome in HNC patients when compared with depleted SMM, illustrating the inadequacy of BMI as an accurate method to reflect nutritional status [8]. An assessment method would be needed for rapid clinical implementation, to adequately evaluate BC in HNC patients in order to reveal significant malnutrition, appropriate chemotherapy dose, and to identify high risk patients [8]. Besides questionnaires like Patient-Generated Subjective Global Assessment (PG-SGA) that allow the assessment of the nutritional status, the existing techniques to evaluate nutritional status and/or BC include anthropometric measurements for weight and BMI, measurement of skinfold thickness, biochemical parameters, bioelectrical impedance analysis (BIA), computed tomography (CT), magnetic resonance (MRI) or dual-energy x-ray absorptiometry (DEXA) [13].
Many studies have shown the impact of CT image of L3 as the reference method to measure BC2. Chamchod et al. (2016) showed that lean body mass (LBM) estimation for HNC patients, especially post-therapy, should be performed using CT image-based assessment, otherwise, measurement error of >10 kg should be presupposed. However, because all HNC patients do not routinely have this image available, bioelectrical impedance analysis (BIA) has been reported as a method with a good consistency along the treatment [2]. This method is widely used, non-invasive, portable, inexpensive, and feasible to assess BC in humans [14]. It is based on impedance of a low-voltage current passing through the body [14], which can then be used to calculate an estimate of total body water (TBW). Further, TBW can be used to estimate fat-free mass (FFM) by comparing with body weight and body fat [8]. Dual-energy X-ray absorptiometry (DEXA) has gained popularity in quantifying LBM for being non-invasive, carrying low cost and radiation dose, and being able to measure LBM, fat mass, and bone mineral density. However, DEXA values depend on the precision error of the DEXA machine, which may be affected by the diffuse inflammatory changes caused by chemotherapy. It remains an important area for research, because there are no recommendations on this issue, and it is important to understand how chemotherapy may affect precision error, in order to accurately interpret changes in BC [15]. This scoping review was guided by the methodologically rigorous manual by The Joanna Briggs Institute (JBI), for scoping reviews [16], and aimed to synthesise and map the BC changes in HNC patients, which occur during treatment. The main objective was to provide a descriptive overview of what these changes are and how they can be measured. The purpose of a scoping review is to map and examine the existing evidence in literature in a given field, providing an overview, as a preliminary exercise prior to the conduct of a systematic review, regardless of quality of the contributing studies, unless otherwise specified. Therefore, a scoping review does not intend to recommend clinical practices or to provide guidelines [16]. An initial search of the JBI Database of Systematic Reviews and Implementation Reports, MEDLINE and CINAHL demonstrated that there were no systematic reviews, meta-analyses or scoping reviews (published or in progress) on this topic. The objective, inclusion criteria and methods for this scoping review were specified in advance and documented in a protocol [16].
Review question/objective
The purpose of this scoping review was to examine and map the BC changes in HNC patients, under active treatment, and to determine which methods are useful to assess BC in these patients. The current review was guided by the following research questions, built on the ‘PCC’ mnemonic (Population, Concept and Context):
- What is known from the existing literature about the changes in BC in head and neck cancer patients under active oncological treatment?
Two other questions were identified to guide the subsequent steps of the scoping review, and broader complement the research question above.
- Which methods are useful for assessing BC changes in HNC patients under active treatment?
- What are their reported strengths and weaknesses?
Inclusion Criteria
As well as the title and the research question, the eligibility criteriawere built on the ‘PCC’ mnemonic (Population, Concept and Context):
Types of participants
The current review considered HNC patients, aged 18 years or older, who had not been submitted to any training or dietary program.
Concept
This scoping review considered all studies that focused on the BC changes.
Context
This scoping review considered the studies that evaluated the BC changes in the context of treatment, with curative intent. These included antineoplastic agents, chemotherapy, adjuvant chemotherapy, radiotherapy, adjuvant radiotherapy, and adjuvant chemoradiotherapy. Surgical treatment approach was not included. Adjuvant treatment was included, but not when this was surgery alone.
Types of sources
This scoping review considered only published studies, both quantitative and qualitative data, with an abstract available. Due to time constraints, only published studies were considered for the review, retrieved from databases, excluding unpublished studies.
Search strategy
The search strategy aimed to find only published studies, within the last 19 years from 2000 to 2019. A three-step search strategy was conducted on this review.An initial limited search of MEDLINE (via PubMed) and CINAHL Plus with Full Text (via EBSCO) was undertaken through an analysis of the index terms used to describe the articles. A second search using all index terms identified was undertaken across both databases included. Thirdly, the reference lists of all identified reports and articles will be searched for additional studies. Studies published between January 2000 and July 10, 2019 in English, Spanish and Portuguese were considered for inclusion in this review. Initial keywords/search terms were used: head and neck cancer; body composition OR body weight OR body weight change OR body mass index OR fat-free mass OR skeletal muscle; antineoplastic agents OR radiotherapy OR radiotherapy, adjuvant OR chemotherapy, adjuvant OR chemoradiotherapy OR chemoradiotherapy, adjuvant. The search in PubMed provided most articles, and the search are shown in Appendix I. The search strategy conducted in Cinahl Plus with Full Text followed the same strategy mentioned in Appendix I. Search results run in the different databases were consolidated, and duplicated studies were excluded. After the duplicates were removed, two independent reviewers screened the articles to exclude those that do not meet the eligibility criteria identified in the second stage of the protocol, based on the titles and abstracts. For those fulfilling the eligibility criteria, the full-text article was obtained. Disagreements on study eligibility of the sampled articles were discussed between the two independent reviewers. Studies identified from reference lists were assessed for relevance based on their title and abstract.
Extraction of results
Relevant data were extracted from the included studies to address the review question using the template developed in the protocol (Appendix II), as indicated by the methodology for scoping reviews developed by the Joanna Briggs Institute [16]. In accordance with the purpose of scoping reviews, the quality of data extracted was not appraised before inclusion. Two reviewers extracted data independently. Disagreements on study eligibility of the sampled articles were discussed between the two independent reviewers, or with a third reviewer. The data extracted included author(s)/year of publication, aims and purpose of the study, sample size, study design, type of treatment, measurement points and component(s) of BC evaluated, BC assessment methods, main results/findings.
Results
The database searches provided a total of 1180 citations after duplicates were removed. One additional citation was found in the reference list review. A total of 17 papers met the inclusion criteria, based on the titles and abstracts. The full-text, of these 17 citations, were obtained and read, and 5 of them were excluded for the following reasons: only assessing skeletal muscle before treatment (n=1), only assessing phase-angle variations during radiotherapy (n=1), only assessing nutrition status, phase-angle and body weight (n=1), patients received exercise training, during and after treatment (n=2), which may serve as a possible confounding for changes body weight or lean body mass. A total of 12 studies were included in this review. A flowchart showing the study selection process is detailed in Figure 1.
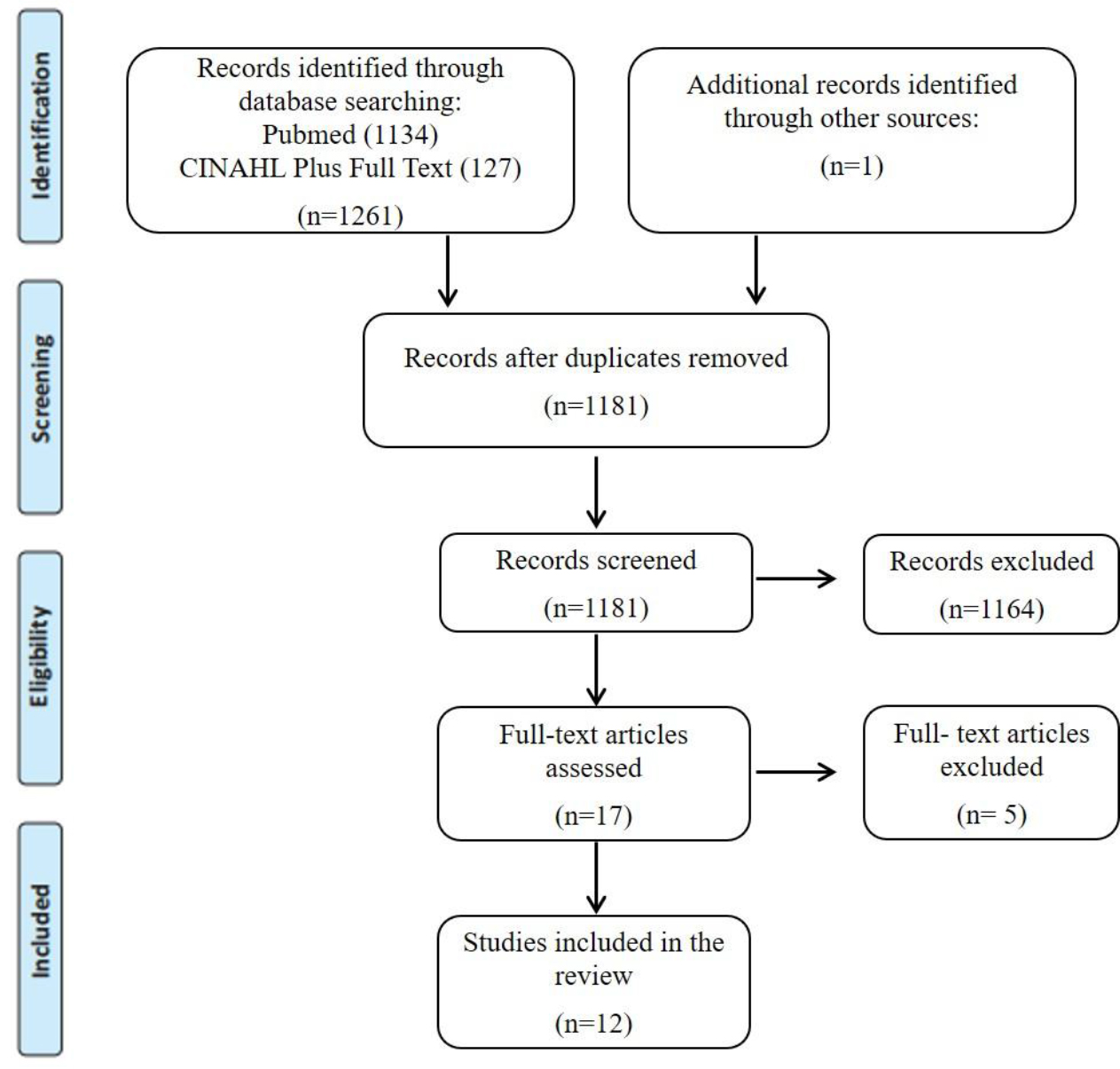
Figure 1. PRISMA flow chart for the scoping review process.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and MetaAnalyses: The PRISMA Statement. PLoS Med 2009; 6 (7): e1000097
Characteristics of Study Design and Data Collection
The review reports found from 12 studies published from 2004 to 2018, had been conducted almost worldwide: China (2) [17, 18] Netherlands (2) [14, 19], United States of America (3) [8, 20, 21] Turkey (1) [22], Brazil (1) [23], Spain (1) [2], Canada (1) [24] and Sweden (1) [25]. A summary of the characteristics of studies included are described in Appendix III. The population size for the included studies ranged from 202to 215 participants [8] comprising a total of 891 HNC patients (75, 3% male; 24, 7% female), over 18 years old. Ten studies had used a prospective cohort design [2, 14, 17, 18-21, 22-25] and two studies had used a retrospective cohort design [8, 20].
Body Composition Changes (Concept)
BC analysis included variables such as: LBM, measured by five studies [8, 17, 19-21] FFM measured by five studies [2, 14, 18, 23, 25] two of which estimated fat-free mass index (FFM (kg) divided by body height2 (m2)) [18, 25]. Fat mass/adipose tissue were measured by seven studies [8, 17, 18, 19, 22-24] one of which estimated fat mass index (FM (kg) divided by body height2 (m2)) [18], and another estimated subcutaneous fat [22].. Skeletal muscle was measured by two studies, normalized for height in meters squared, reported by skeletal muscle index (SMI) [18, 24]. Reviewing the articles and synthesizing findings from baseline to the end of treatment, all studies reported BC changes, especially loss of LBM or FFM. Appendix IV shows the BC changes reported from the baseline to the end of treatment. The BC analysis data collected at baseline were set as the reference to analyses whether significant changes were observed at different measurement points. One study [2] reported a positive change in FFM during induction chemotherapy (iCT), which increased until the begin of concomitance treatment, and then declined significantly, while another study [21] reported also a positive change during iCT, but this was related to weight gain and not specifically to FFM. The greatest change in body mass occurred in LBM at 1-month post-concurrent chemoradiotherapy (CRT). The loss in LBM occurred despite dietary consumption, and reduced significantly for all body compartments: arms, legs, and trunk [21]. One study [25] reported a decrease of FFM of 6, 5 kg in >10% weight loss group, and 2, 7 kg in ≤ 10% weight loss group. One study [24] including 28 HNC patients found that approximately half of lost body weight was attributed specifically to muscle loss (3.4 kg) and the other half could be explained by 3.6 kg fat loss, both visceral and subcutaneous adipose tissue. The same results were reported in another the study [19], where LBM significantly declined during treatment, corresponding a sixty-two percent of weight loss. However, there were no significant changes between first and second post treatment assessment, which is in agreement with the results found by the same authors, but in a more recent study14 showing a significant decline in FFM (p<.05) during the treatment period but remaining stable 4 months after the end of treatment. One study [17] reported a significant decline in body fat mass and LBM at the different time points after radiotherapy (RT) compared with pre-RT. During the recovery time from the end of treatment to 6 months post-RT, lean body mass remained largely static, whereas body fat mass continued to decrease. Two studies [7, 20] reported different results by sex. In men, a significantly dropped of LBM post-treatment compared with pre-treatment was found, decreased from @ 58kg to @ 51kg after RT, whereas mean estimated LBM in women remained fairly stable, decreasing from @ 38.0 kg to @ 36 kg RT. Additionally, another study [8] reported that the mean fat mass dropped in both men and women after treatment. Two studies [22, 23] showed that TST measurements significantly deteriorated in the end of RT, what means a significant loss of subcutaneous fat, corroborated by BIA analysis, which demonstrated a significant reduction in body fat and FFM, which continued to decline one month after the end of treatment [23]. One study [18] reported statistically and clinically significant changes also in fat mass, FFM, and SMM, during concurrent CRT. Synthesizing these findings shows how HNC patients suffer from serious LBM, skeletal muscle, body fat or FFM loss, during and after treatment compared with baseline.
Body composition assessment methods
In five studies [2, 14, 18, 23] BC was assessed by BIA, two of which also used DEXA [14] or TSF [23], respectively. In three studies [8, 20, 24] the assessment method was CT, at the level of the third lumbar (L3) vertebra. DEXA, as a single measurement, was applied in other three studies [17, 19, 21] and in one study [22] BC was assessed by TSF. One study [2] considered BIA as a method with good application consistency in patients with locally advanced HNC providing useful information, especially for evaluating FFM, since these patients do not have image of L3 at the CT available in a regular daily basis. However, also referred that the validity and interpretation of maintenance in FFM through the treatment in his study, need to be taken cautiously as the BC values were estimated from changes in voltage across the body. In another study [17] on nasopharyngeal cancer patients managed in Hong Kong, the authors did not find a systematic difference between BIA and DEXA measures, although the BIA had slightly underestimated FFM by <1 kg, both pre- and post-treatment, and accordingly to these results, one study [18] in locally advanced nasopharyngeal carcinoma patients showed that BC assessed by BIA could reflect the change of nutritional status when compared with other methods such as DEXA. One study [8] including HNC patients (various tumor subsites) recommended routine use of quantitative imaging (CT and DEXA) in HNC patients, especially in those prone to changes in nutritional status, as opposed to general population-based height-weight formulae, because the last are not sufficient for body mass quantification. One study [22] used triceps skinfold thickness (TSF) to estimate subscutaneous fat in a series of 54 HNC patients. This is a inexpensive and non-invasive method, and it is widely available [13]. On the other hand, eight studies [17, 19-25] did not report any advantage or disadvantage of using BIA, L3 image at CT, TSF or DEXA. In summary, these findings show that BIA has the great advantage for being available on a regular basis for assessing BC in HNC patients, is inexpensive, noninvasive, and it is a good method to be applied when no imaging techniquesare available. Further, it can be performed by a clinical dietitian [18] if the protocol is followed.
Discussion
This scoping review report’s findings from 12 articles identified through a systematic literature search, published over a 14-year period, that investigated or described the BC changes in HNC patients under active oncological treatment, with curative intent. The patient group with surgical treatment approach was not included in this review for uniformity reasons, as this would have increased the heterogenous nature of the patient population. In general, the studies displayed different oncological treatment modalities, the sample sizes in the retrospective studies suffer from dropout, and the prospective studies from small samples. The studies included in this scoping review also comprised different ‘’measurement points’’, evaluated different components of BC, as well as the methods to assess it in HNC patients, which makes it challenge to synthesizing findings. Studies of human BC using CT scans have provided proof-of-concept that variability in drug disposition and toxicity profiles may be partially explained by different features in BC [26]. The depletion of skeletal muscle before and after RT is strongly associated with decreased survival in patients with solid tumors [20], a higher risk for complications and reduced response to cancer treatment [19]. BC analysis results indicated that the BC components, such as LBM, FFM, body fat and skeletal muscle, change at different measurement points, and that these changes in HNC patients, receiving RT, cannot be effectively monitored by measuring their weight, and BMI [27].. Two studies [19, 24] reported a loss of LBM corresponding to more than half the weight lost, showing that weight loss itself poorly predicts outcome in HNC patients [8]. Low dietary intake due to treatment related nutrition impact symptoms seems to be one of the main contributing factors for muscle loss in HNC patients, because they not meet the recommended calorie and protein intake. In additional to low dietary intake, inflammation could exacerbate muscle loss during cancer treatment [24], as well as impairments in physical performance, contributing to aberrant changes in BC [20]. However, there was a positive change in FFM during iCT [2], reported by Arribas et al. (2017), which may be related to the improvement of the symptoms that initially limited the oral intake and could contribute to minimize further deterioration, proving the role of the nutritional intervention from the beginning of the treatment [2]. Besides these two studies, and this specific measurement point, all the studies included in this review reported loss of LBM, FFM, fat mass and skeletal muscle during the treatment. Post-treatment nutritional deterioration is evident among HNC patients in all included studies, occurring up to 8-12 months during follow-up, although there appears to be a slight recovery. Different findings were observed between Jager-Wittenaar et al (2010) and Kenway et al. (2004) related to body-weight increase after treatment. Jager-Wittenaar et al. (2010) reported a weight gain, characterized by increase of fat mass instead of FFM, while Kenway et al. (2004) found a continously decline of body fat after treatment. Changes in BC after cancer treatment warrant further investigation as this phenomenon might affect recovery from therapy-related side effects and more importantly, even prevent complications. A systematic review by Correia et al.(2019) addressing the methods for BC assessment in clinical settings found that the reference methods for BC assessment in cancer patients are DEXA and L3 in CT imaging, but these examinations are not routinely performed in the management of HNC. This finding is consistent with this review where some authors chose BIA as the preferred method as an alternative to more invasive and expensive methods like DEXA and CT, and because it is available on in routine HNC management. BIA is recommended to be increasingly implemented in nutritional assessment [18]. Citak et al. (2017) used TSF to estimate subscutaneous fat, and only highlight advantages for its use, because all anthropometric measurements were performed by the same person [22]. However, poorer accuracy and precision in obese/oedematous individuals [28], and its sensitive to technician skills, type of calliper and prediction equations used it [13], need to be taken into account. All the BC changes that occur during management of HNC patients, as well as choosing the most feasible, accurate and practical method to assess these changes, represents a challenge for further investigation, in order to assess and improve nutritional status, and disease-associated processes.
Limitations of the review
Although the quality of data extracted was not appraised before inclusion, since it is not relevant for a scoping review, some limitations should be reported so as to provide valuable information to future investigation:
The present scoping review is a pragmatic mean of dealing with the lack of evidence available on BC changes in HNC patients under active treatment;
- The present scoping review aims to use 2 electronic databases and the search has been refined to increase the likelihood of retrieving as many relevant published articles as possible;
- Only published studies, in English, Portuguese and Spanish in scientific journals were considered eligible for inclusion;
- A quality assessment of the articles included in the scoping review was not performed;
- Due to time constraints, the search strategy didn’t include the MeSH term “neoplasms”, what may had excluded some relevant references;
- The difference in results may be the result of including a heterogeneous group of patients receiving different types of treatment, and of the variability of BC assessment tools;
- The interval of BC assessment between pre- and post- RT varied, and some patients may have recovered muscle mass during this period whereas other may have continued to lose muscle mass after the end of treatment;
- The variability in quality of imaging, which may affect skeletal muscle mass, contouring and adipose tissue segmentation.
Conclusion
HNC patients experience a significant depletion of LBM, FFM and skeletal muscle, accompanied by body fat mass, while undergoing (chemo) radiotherapy, demonstrated either by the TSF, BIA, DEXA or CT. This loss has a significant impact on their survival, quality of life, on the risk for complications and may result in a reduced response to cancer treatment. Thus, BC assessment should become an integral component of the care of HNC patients, beyond weight and BMI, and should be carried out at different times throughout treatment. Based on this review, further investigations are recommended applying measurements at same time points and assessing BC changes with comparable methods in order to obtain evidence for the impact of body composition changes in this patient population.
Appendix I – Search Strategy
PubMed – search conducted on 10/07/2019
|
Search Strategy |
Results |
|
(((“Head and Neck Neoplasms”[Mesh] OR (head[Title/Abstract] AND Neck neoplasms[Title/Abstract])) OR (Head[Title/Abstract] AND neck cancer[Title/Abstract])) AND (((((((((“Muscle, Skeletal”[Mesh] OR (“muscle, skeletal”[MeSH Terms] OR (“muscle”[All Fields] AND “skeletal”[All Fields]) OR “skeletal muscle”[All Fields] OR (“muscle”[All Fields] AND “skeletal”[All Fields]) OR “muscle, skeletal”[All Fields])) OR (“Adipose Tissue”[Mesh] OR Adipose Tissue[Title/Abstract])) OR (“Adiposity”[Mesh] OR Adiposity[Title/Abstract])) OR (“Body Composition”[Mesh] OR body composition[Title/Abstract])) OR (“Body Mass Index”[Mesh] OR Body Mass Index[Title/Abstract])) OR (“Weight Loss”[Mesh] OR weight Loss[Title/Abstract])) OR (“Weight Gain”[Mesh] OR Weight Gain[Title/Abstract])) OR (Body Weight Changes[Title/Abstract] OR “Body Weight Changes”[Mesh])) OR (“Body Weight”[Mesh] OR Body Weight[Title/Abstract]))) AND ((((((“Chemoradiotherapy, Adjuvant”[Mesh] OR Adjuvant Chemoradiotherapy[Title/Abstract]) OR (“Chemoradiotherapy”[Mesh] OR Chemoradiotherapy[Title/Abstract])) OR (“Radiotherapy, Adjuvant”[Mesh] OR Adjuvant Radiotherapy[Title/Abstract])) OR (“Chemotherapy, Adjuvant”[Mesh] OR Adjuvant Chemotherapy[Title/Abstract])) OR (“Radiotherapy”[Mesh] OR Radiotherapy[Title/Abstract])) OR (“Antineoplastic Agents”[Mesh] OR Chemotherapy[Title/Abstract])) AND (hasabstract[text] AND (“2000/01/01”[PDAT] : “3000/12/31”[PDAT]) AND “humans”[MeSH Terms] AND (English[lang] OR Portuguese[lang] OR Spanish[lang])) |
1134 |
Appendix II: Data extraction instrument
|
Scoping Review Title: Body composition changes in head and neck cancer patients under active treatment: a scoping review Review Objective/s: Examine and map the body composition changes in head and neck cancer patients, under active treatment, and determine which methods are used to assess body composition in these patients. Review Question/s:
Inclusion/Exclusion Criteria Population: Head and neck cancer patients, aged 18 years or older, who have not been submitted to any training or dietary program. Concept: Body composition changes. Context: Treatment: This include antineoplastic agents, chemotherapy, adjuvant chemotherapy, radiotherapy, adjuvant radiotherapy, chemoradiotherapy and adjuvant chemoradiotherapy. Types of Study: Only published studies, both quantitative and qualitative data, and systematic reviews, with abstract available. Study Details and Characteristics Author/Year_____________________________________________________________ Aims/Purpose of the study__________________________________________________ Sample Size_____________________________________________________________ Study design_____________________________________________________________ Type of treatment_________________________________________________________ Measurement points_______________________________________________________ Component(s) of body composition evaluated___________________________________ Body composition assessment method_________________________________________ Main results/findings_______________________________________________________ |
Appendix III: C haracteristics of Study Design and Data Collection
|
Author(s)/ Year of publication |
Aims/ Purpose of the study |
Sample Size/Stage |
Study Design |
Type of treatment |
Measurement Points |
Component(s) of body composition evaluated |
Body composition assessment method |
Main results/findings |
|
Arribas et al2 2017 |
To evaluate changes in BC and nutritional status that occur throughout the oncological treatment in HNC patients |
N = 20 HNSCC (Men = 19; women = 1) |
Prospective cohort study |
iCT flollowed by CRT or RT plus Cetuximab |
Baseline Post iCT; After RT; 1 month post RT; 3 months post RT |
Fat Free Mass |
BIA |
FFM decrease significantly over the course of treatment, but after the iCT there was an increase in FFM |
|
Silander et al25 2012 |
To identify predictors of malnutrition at time of diagnosis in order to identify patients at risk and enable early nutritional support and prevent malnutrition |
N = 119 Pharyngeal cancer, oral cancer, or unkwon primary with malignant neck nodes in stage III ou IV (Men = 81; women = 38) |
Prospective cohort study |
Chemotherapy and RT, or surgery followed by RT |
Baseline; After 5 months follow up |
Fat Free Mass |
BIA |
The decrease of FFM was more than twofold for the malnourished patients, applying a definition of >10% weight loss compared to the non-malnourished during the 6-month time period, 6.5 kg versus 2.7 kg |
|
Nejatinamini et al24 2018 |
To assess changes in vitamin status during HNC treatment in relation to BC, inflammation and mucositis |
N = 28 HNSCC of the oral cavity, pharynx and larynx (Men = 23; women = 5) |
Prospective cohort study |
RT, with or without chemotherapy |
Baseline; Post treatment (=6 months) |
Skeletal muscle and body fat |
Computed tomography at the level of the third lumbar (L3) vertebra |
Approximately half of lost body weight was attributed specifically to muscle loss (3.4 kg) and the other half could be explained by 3.6 kg fat loss. Patients experienced a significant decrease in both visceral and subcutaneous adipose tissue |
|
Kenway et al17 2004 |
To investigate the nutritional status of NPC patients before and after RT and the factors affecting nutritional by examining the relation among changes in body weight, BC, and basal metabolic rate; calorie intake; and total energy expenditure and adjusting for tumor stage, patient age, and gender |
N = 38 Nasopharynx cancer (Men = 30; women = 8) |
Prospective cohort study |
Radiotherapy |
Baseline; post-RT; 2 months post RT; 6 months post RT |
Body Fat and lean body mass |
DEXA |
Body fat mass and lean body mass at the different time points post-RT were all significantly lower than that at pre-RT. During the recovery time from end-RT to 6 months post-RT, lean body mass remained largely static, whereas body fat mass continued to decrease |
|
Jager-Wittenaar eta I19 2010 |
To test whether nutritional status (including body weight, lean mass, and fat mass) of patients with head and neck cancer changes during and after treatment |
N = 29 HNSCC of the oral cavity, pharynx and larynx (Men = 23; women = 6) |
Prospective cohort study |
Radiotherapy, either alone or combined with chemotherapy or surgery |
1 week before treatment; 1 month posttreatment; 4 months posttreatment |
Lean body mass and fat mass |
DEXA |
Body weight, BMI, and lean mass significantly declined during treatment. Sixty-two percent of weight loss was loss of lean mass. Lean mass declined significantly in all body regions |
|
Grossberg et al20 2016 |
To determine whether lean body mass before and after RT for HNSCC predicts survival and locoregional control |
N = 190 HNSCC (Men = 160; women = 30) |
Retrospective cohort study |
Primary surgery, single-modality RT or concurrent CRT |
Before and after RT (= 8-12 months) |
Lean body mass |
Computed tomography at the level of the third lumbar (L3) vertebra |
In Men, mean estimated LBM decreased from 58.4kg to 51.6kg after RT, whereas mean estimated LBM in women remained fairly stable, decreasing from 38.0 kg to 35.7 kg after RT |
|
Citak et al22 2017 |
To assess the nutritional status and to define its determinants in patients with HNC undergoing RT |
N = 54 HNC (Men = 49; women = 5) |
Prospective cohort study |
RT, with or without chemotherapy, after surgery, or not. |
Baseline After RT; 1 month post RT; 3 months post RT |
Subcutaneous fat |
Triceps Skinfold Thickness (TST) |
TST measurements were significantly deteriorated in the end of RT |
|
Silver et al21 2006 |
To determine changes in body mass and BC in relation to energy balance, inflammatory state, and physical function before and after concurrent CRT |
N = 70 HNSCC of the oral cavity, hipopharynx and larynx (Men = 15; women = 55) |
Prospective cohort study |
Concurrent CRT |
Baseline; 1 month post CRT |
Lean body mass |
DEXA |
The greatest change in body mass occurred in LBM at 1- month postconcurrent CRT. The loss in LBM occurred despite dietary consumption, and reduced significantly for all body compartMents: arms, legs, and trunk. |
|
Carvalho et al23 2013 |
To examine the involveMent of antitumor treatment, including surgical resection and/or CRT, in the nutritional and metabolic status of patients with SCCHN |
N= 32 HNSCC (Men = 31; women = 30) |
Prospective cohort study |
CRT, with or without previously surgery |
10 to 20 days before the beginning of CRT; 30 to 40 days after finishing CRT |
Body fat and FFM |
BIA |
There was significant reduction in body fat and FFM. The weight loss was accompanied by a significant reduction in body fat percentage calculated from TST and BIA |
|
Ding et al18 2018 |
To investigate BC changes in patients with nasopharyngeal carcinoma undergoing concurrent CRT and a comparison of the Patient-Generated Subjective Global assessment (PG_SGA) and the ESPEN (European Society for Clinical Nutrition and Metabolism) diagnostic criteria as evaluation methods. |
N = 48 Nasopharyngeal Carcinoma (Men = 36; women = 12) |
Prospective cohort study |
Concurrente CRT, with or without iCT |
Baseline; weekly until the end of treatment |
Fat mass, FFM and skeletal muscle |
BIA |
During concurrent CRT, there were statistically and clinically significant changes in most BC parameters, including body cell mass, fat mass, FFM, and SMM, as well as body weight, BMI, and PG-SGA scores |
|
Chamchod et al8 2016 |
To determine if one or more height-weight formula(e) can be clinically used as a surrogate for direct CT- based imaging assessment of BC before and after RT for HNC patients, who are at risk for cancer and therapy- associated cachexia/sarcopenia. |
N = 215 HNC (Men = 184; women = 31) |
Retrospective cohort study |
Concurrent chemotherapy or RT, with or without surgery |
Pre- and posttreatment |
Lean body mass |
Computed tomography at the level of the third lumbar (L3) vertebra |
Mean LBM dropped significantly posttreatment compared to pre-treatment for Men but didn’t reach statistical significance in women. Additionally, mean fat mass dropped in both Men and women after treatment |
|
Jager-Wittenaar et al14 2014 |
To validate BIA using the Geneva equation for FFM in HNC patients |
N = 24 HNC (Men = 20; women = 4) |
Prospective cohort study |
RT, with or without chemotherapy, or after surgery |
The week before the treatment; 1 month after treatment; 4 months treatment |
Fat Free Mass |
DEXA and BIA |
Body weight, BMI, FFM, volume of body fluids, phase angle, and impedance ratio significantly declined during the treatment period . There was no systematic difference between the BIA and DXA measurements. |
Appendix IV: BC changes reported from the baseline to the end of treatment
|
Author(s) |
Body composition assessment method |
Component(s) of body composition evaluated |
Baseline |
Post iCT |
Post RT |
1-2 months post treatment |
3-4 months post treatment |
5-6 months post treatment |
8-12 months post treatment |
|
Arribas et al2 |
BIA |
Fat free mass (kg)* |
53,69 (8,16) |
55,96 (9,06) |
51,54 (5,89) |
52,08 (6,70) |
50,05 (7,66) |
|
|
|
Silander et al25 |
BIA |
Fat free mass index* |
(2,6) |
|
|
|
|
– 2,7 kg FFM (WL < 10% group) -6,5 kg FFM (WL > 10% group) |
|
|
Nejatinamini et al24 |
Computed |
Skeletal muscle index (cm2/m2)* |
52,6 (11,1) |
|
|
|
|
45,5 (9,1) |
|
|
tomography |
Body fat (kg)* |
28,3 (8,1) |
|
|
|
|
24,7 (6,9) |
|
|
|
Kenway et al17 |
DEXA |
Lean body mass (kg)* |
46,2 (8,3) |
|
42,1 (7,8) |
42,8 (7,6) |
|
43,3 (7,5) |
|
|
Body fat (kg)* |
15,1 (4,9) |
|
12,4 (4,7) |
10,9 (3,8) |
|
10,4 (3,5) |
|
||
|
Jager-Wittenaar |
DEXA |
Lean body mass (kg)* |
54,6 (11,4) |
|
|
52,1 (10,7) |
52,3 (10,3) |
|
|
|
et al 19 |
Body fat (kg)* |
20,0 (9,8) |
|
|
18,9 (8,1) |
19,0 (7,0) |
|
|
|
|
Grossberg et al20 |
Computed tomography |
Lean body mass (kg)* |
58,4 (9,6) (men) 38,0 (7,3) (women) |
|
|
|
|
|
51,6 (7,8) (men) 35,7 (6,6) (women) |
|
Citak et al22 |
Triceps Skinfold Thickness (TST) |
Subcutaneous fat (mm)* |
21,79 (4,47) |
|
21,31 (3,98) |
21,5 (3,93) |
21,81 (4,00) |
|
|
|
Silver et al21 |
DEXA |
Lean body mass (kg)* |
52,25 (11,33) |
|
|
46,64 (96,52) |
|
|
|
|
Carvalho et al23 |
Triceps Skinfold Thickness (TST) |
Subcutaneous fat (mm)* |
10,84 (5,78) |
|
|
8,41 (4,56) |
|
|
|
|
BIA |
Body fat (%)* |
27,27 (7,44) |
|
|
23,36 (7,70) |
|
|
|
|
|
|
Fat free mass (kg)* |
48,30 (9,84) |
|
|
45,90 (8,75) |
|
|
|
|
|
|
|
Fat mass index (kg2/m2)* |
7,66 (1,99) |
|
6,61(1,87) |
|
|
|
|
|
Ding etal18 |
BIA |
Fat free mass index (kg2/m2)* |
15,79 (1,82) |
|
14,79 (2,02) |
|
|
|
|
|
|
|
Skeletal muscle (kg)* |
24,47 (6,01) |
|
22,93 (5,86) |
|
|
|
|
|
Chamchod et al8 |
Computed |
Lean body mass (kg)* |
58,0 (9,69) (men) 37,56 (7,0) (women) |
|
51,52 (8,31) (men) 36,2 (7,13) (women) |
|
|
|
|
|
tomography |
Body fat (kg)* |
18,48 (1,36) (men) 17,56 (1,16) (women) |
|
15,61 (0,98) (men) 15,42 (1,00) (women) |
|
|
|
|
|
|
Jager-Wittenaar |
DEXA |
Fat Free Mass (kg)* |
56,4 (10,9) |
|
|
54,2 (10,0) |
54,4 (9,9) |
|
|
|
et al14 |
BIA |
55,7 (10,0) |
|
|
53,9 (9,4) |
54,4 (9,4) |
|
|
|
|
* Mean, standard deviation WL-Weigh Loss |
|||||||||
References
- Govender R, Smith CH, Taylor SA, Grey D, Wardle J et al. (2015) Identification of behaviour change components in swallowing interventions for head and neck cancer patients: protocol for a systematic review. Systematic Reviews 4: 89. [crossref]
- Arribas L, Hurtós L, Taberna M, Peiró I, Vilajosana E et al. (2017) Nutritional changes in patients with locally advanced head and neck cancer during treatment. Oral Oncology 71: 67–74. [crossref]
- Yanni A, Dequanter D, Lechien JR, Loeb I, Rodriguez A et al. (2019)Malnutrition in head and neck cancer patients: Impacts and indications of a prophylactic percutaneous endoscopic gastrostomy. Eur Ann Otorhinolaryngol Head Neck Dis136: 27–33.[crossref]
- Formigosa JAS, Costa LS, Vasconselos EV (2018) Social representations of patients with head and neck cancer before the alteration of their body image. Rev Fund Care Online 10: 180–189.
- Stenson KM, MacCracken E, List M, Haraf DJ, Brockstein B et al.(2000) Swallowing function in patients with head and neck cancer prior to treatment. Arch Otolaryngol – Head Neck Surg 126: 371–377. [crossref]
- Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H et al. (2017) ESPEN guidelines on nutrition in cancer patients. ClinNutr 36: 11–48. [crossref]
- Van Liew J, Brock R, Christensen A, Karnell, L, Pagedar N et al. (2017) Weight loss after head and neck cancer: A dynamic relationship with depressive symptoms. Head Neck 39: 370–379. [crossref]
- Chamchod S, Fuller C, Mohamed A, Grossberg A, Messer J et al. (2016) Quantitative Body Mass Characterization Before and After Head and Neck Cancer Radiotherapy: A Challenge of Height-Weight Formulae Using Computed Tomography Measurement. Oral Oncology 61: 62–69. [crossref]
- Marshall KM, Loeliger J, Nolte L, Kelaart A (2018) Prevalence of malnutrition and impact on clinical outcomes in cancer services: A comparison of two time points. ClinNutr 38: 1–8. [crossref]
- Couch ME, Dittus K, Toth MJ, Willis M, Guttridge D et al. (2015) Cancer cachexia update in head and neck cancer: Definitions and diagnostic features. Head Neck 37: 594–604. [crossref]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E et al. (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12: 489–495. [crossref]
- Deluche E, Leobon S, Desport JC, Venat-Bouvet L, Usseglio J et al. (2018) Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 26: 861–868. [crossref]
- Correia I, Neves P, Makitie A, Ravasco P (2019) Body Composition evaluation in Head and Neck Cancer Patients: A Systematic Review (2019) Under Review in Frontiers in Nutrition.
- Jager-Wittenaar H, Dijkstra PU, Earthman CP, Krijnen W, Langendijk J et al. (2014) Validity of bioelectrical impedance analysis to assess fat-free mass in patients with head and neck cancer: an exploratory study. Head Neck 36: 585–591. [crossref]
- Chang HC, Lin YC, Ng SH, Cheung YC, Wang CH et al. (2019) Effect of Chemotherapy on Dual-Energy X-ray Absorptiometry (DXA) Body Composition Precision Error in Head and Neck Cancer Patients. J Clin Densitom 22: 437–443. [crossref]
- Peters MD, Godfrey CM, McInerney P, Soares CB, Khalil H et al. (2015) Joanna Briggs Institute Reviewers’ Manual 2015: Methodology for JBI Scoping Reviews. South Australia: The Joanna Briggs Institute.
- Ng K, Leung SF, Johnson PJ, Woo J (2004) Nutritional Consequences of Radiotherapy in Nasopharynx Cancer Patients. Nutrition and Cancer 49: 156–161. [crossref]
- Ding H, Dou S, Ling Y, Zhu G, Wang Q et al. (2018) Longitudinal Body Composition Changes and the Importance of Fat-Free Mass Index in Locally Advanced Nasopharyngeal Carcinoma Patients Undergoing Concurrent Chemoradiotherapy. Integr Cancer Ther 17: 1125–1131. [crossref]
- Jager-Wittenaar H, DijkstraPU, Vissink A, Langendijk JA, van der Laan BF et al. (2010) Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head & Neck 33: 863–870. [crossref]
- Grossberg AJ, Chamchod S, Fuller CD, Mohamed ASR, Heukelom J et al. (2016) Association of body composition with survival and locoregional control of radiotherapy-treated head and neck squamous cell carcinoma. JAMA Oncol 2: 782–789. [crossref]
- Silver HJ, Dietrich MS, Murphy BA (2007) Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head & Neck 29: 893–900. [crossref]
- Citak E, Tulek, Z, Uzel O (2018) Nutritional status in patients with head and neck cancer undergoing radiotherapy: a longitudinal study. Supportive Care in Cancer. Support Care Cancer 27: 239–247. [crossref]
- De Carvalho TMR, Miguel Marin D, da Silva CA, de SouzaAL, Talamoni M (2014) Evaluation of patients with head and neck cancer performing standard treatment in relation to body composition, resting metabolic rate, and inflammatory cytokines. Head & Neck 37: 97–102. [crossref]
- Nejatinamini S, Debenham BJ, Clugston RD, Mawani A, Parliament M et al. (2018) Poor vitamin status is associated with skeletal muscle loss and mucositis in head and neck cancer patients. Nutrients 10. [crossref]
- Silander E, Nyman J, Hammerlid E (2013) An exploration of factors predicting malnutrition in patients with advanced head and neck cancer. Laryngoscope 123: 2428–2434. [crossref]
- Prado CMM (2013) Body composition in chemotherapy: The promising role of CT scans. CurrOpinClinNutrMetab Care 16: 525–533. [crossref]
- Tang PL, Wang HH, Lin HS, Liu WS, Chen LM et al. (2018) Body Composition Early Identifies Cancer Patients with Radiotherapy at Risk for Malnutrition. Journal of Pain and Symptom Management 55: 864–871. [crossref]
- Wells JCK, Fewtrell MS (2006) Measuring body composition. Arch Dis Child 91: 612–617.