Abstract
Cervical cancer is the second most common leading cause of cancer death among women worldwide. Annually, ≥ 300,000 women die of cervical cancer and the majority of these deaths occur in developing regions of the world including sub-Sahara Africa. Human papillomavirus (HPV) is necessary but not a sufficient cause of cervical cancer. This review paper evaluated risk factors associated with cervical cancer in sub-Saharan Africa, using recent epidemiological studies. The main risk factors associated with cervical cancer in the sub-Saharan African women were; infection with high-risk HPV subtypes, HIV infection, socio-economic factors, age at first sexual intercourse, multiple sexual partners, and long-term use of oral contraceptives. Multi-parity, early pregnancies, and cigarette smoking are some of the risk factors associated with an increased risk of cervical cancer in sub-Saharan Africa. In addition, candidate gene studies have identified a number of single nucleotide polymorphisms mainly within the immune response genes to be associated with cervical cancer risk. Recently, dysbiosis of the cervical microbiome has been associated with cervical cancer risk in sub-Saharan African women.
Keywords
Cervical cancer, Incidence, Sub-Saharan Africa, HPV, Risk Factors
Introduction
Cervical cancer is the second most common leading cause of cancer death among women worldwide [1]. More than 300,000 cervical cancer deaths are reported annually and the majority of these deaths are from developing regions of the world. Squamous cell carcinoma (SCC) and adenocarcinoma (ADC) are the main histological types of cervical cancer [2,3]. SCC begins in epithelial cells of the ectocervix while ADC develops in the glandular cells that line the endocervix [4]. Approximately 80% and 20% of all cervical cancers are SCC and ADC, respectively [5]. ADC is more aggressive and frequently have distant metastases. Patients with ADC tend to have lower five-year survival rates and require an alternative approach to treatment than those with SCC [6]. This review paper evaluates the incidence and risk factors associated with cervical cancer in sub-Saharan Africa, using recent evidence from epidemiological studies.
Incidence of cervical cancer
The incidence of cervical cancer has been decreasing steadily for the past three decades in industrialized regions of the world [7]. However, in developing regions of the world especially in sub-Saharan Africa, the incidence of cervical cancer is increasing at an alarming rate [8]. The age-standardized incidence rate (ASIR) of cervical cancer in sub-Saharan Africa was estimated to be 34.9/100, 000 women [1]. Moreover, ASIR of cervical cancer varies greatly within sub-Saharan Africa, with ASIRs of 43.4/100, 000 women reported in southern Africa, 40.1/100, 000 women in eastern Africa, 29.6/100, 000 women in western Africa, and 26.8/100, 000 women in middle Africa [1], (Figure 1). According to the global cancer statistics of 2018, Eswatini has the highest incidence of cervical cancer followed by Malawi. The ASIRs of cervical cancer in Eswatini and Malawi are 75.3/100, 000 and 72.9/100, 000 women, respectively. Moreover, it is estimated that in the absence of any intervention, nearly 16.5 million cervical cancer cases will occur in sub-Saharan Africa in the next five decades [9].
Risk factors for cervical cancer
1. Human papillomavirus (HPV) infection
HPV is necessary but not sufficient cause of cervical cancer [10]. Approximately 90% of cervical cancers are attributed to HPV infection [11]. HPV, a small, non-enveloped, double-stranded circular DNA viruses belong to the Papovaviridae family [12]. There are ≥150 HPV subtypes that have been identified and characterized so far. These subtypes are categorized into high and low-risk according to their ability to cause malignant tumours. The high-risk HPV subtypes include 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68, 69, 73, and 82 [13]. Within this category, HPV subtypes 16 and 18 are the most oncogenic and contribute ≥ 70% of all cervical cancer cases [14].
Over 90% of HPV is cleared by the immune system within two years after infection [15]. However, a small proportion of infections especially those with high-risk HPV subtypes can persist and progress to cervical cancer. HPV encodes eight early viral regulatory protein (E1 to E8) and two late structural proteins (L1 and L2), which are crucial for cervical carcinogenesis [16–18]. The E1 and E2 are required for viral DNA replication. The E2 suppresses E6 and E7 viral oncoprotein, E4 and E5 help viral assembly, whereas L1 and L2 are involved in capsid formation [19]. HPV usually integrate its DNA into the human genome for replication. However, the integration of HPV into the human DNA disrupts the E2 functionality, thus resulting in a higher expression of E6 and E7 oncoprotein [20]. These oncoproteins invade the host immune system, deregulate the cell cycle control and apoptosis, thus allowing viral persistence. Dysregulation of the cell cycle and apoptosis lead to cellular transformation and immortalization, which is an important step in cervical tumorigenesis [19]. Specifically, E6 bind to tumour suppressor gene (TP53) and prevents apoptosis, whilst E7 oncoprotein promotes cellular proliferation and differentiation [21].
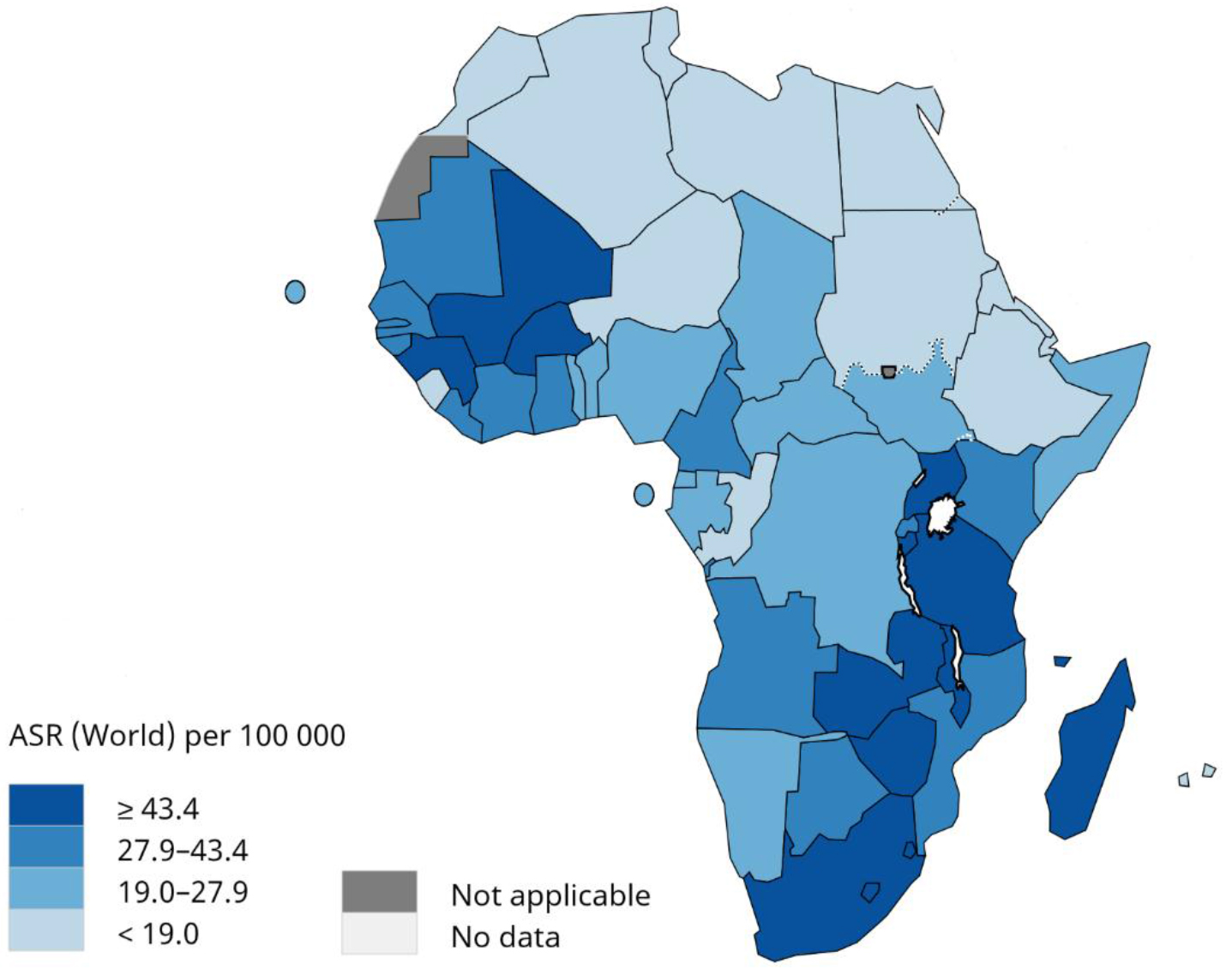
Figure 1. Age-standardized incidence rate of cervical cancer in sub-Saharan Africa. Adapted from Global cancer statistics 2018, International Agency for Research on Cancer, World Health Organization.
A retrospective study from 38 countries in North America, Latin America, Caribbean, Europe, Africa, Asia, and Oceania revealed that ≥ 85% of cervical cancer tissues were HPV positive [11]. In addition, 70% of cervical cancer cases were reported to be caused by HPV subtype 16 and 18 [22]. Studies have suggested that in addition to high-risk HPV subtypes been associated with cervical cancer, certain low-risk HPV subtypes such as subtype 6 and 11 also play a crucial role in cervical carcinogenesis [23–25]. However, these low-risk HPV subtypes are commonly associated with benign genital warts. Epidemiological studies have confirmed the direct role of several HPV subtypes in the development of cervical cancer [26,27]. Persistent infection with high-risk HPV subtypes has also been implicated in other cancers including that of the anus, penis, vulva, vagina, and oropharynx [10,28]. These findings suggest that HPV is not only associated with cervical cancer but also other cancers.
2. Human immunodeficiency virus (HIV) infection
HIV exacerbates the risk of cervical cancer. Population-based studies have reported that HIV-positive women are more likely to develop cervical cancer than HIV-negative women [29,30]. In Senegal, HIV-positive women were 2.55, (95% CI 1.69–3.86) times more likely to progress from HPV infection to cervical cancer than HIV-negative women [31]. A case-control in Eswatini reported that HIV positive women were 5.24 times increased risk of cervical cancer than HIV negative women [32]. The increased risk of cervical cancer among HIV-positive women is particularly important in sub-Saharan Africa, where HIV infection is endemic. In Rwanda, Singh et al. reported a higher prevalence of high-risk HPV subtypes in HIV-positive women than in HIV-negative women [33]. In Zambia, HIV-positive individuals were two-timed more likely to be co-infected with high-risk HPV subtypes than HIV-negative individuals [34]. In Zimbabwe and South Africa, HIV-positive women were more likely to have abnormal cervical cytology than HIV-negative women [35,36]. A number of studies have indicated that HIV infection suppresses the immune system’s ability to clear the HPV infection [37–39], leading to persistent infection, which subsequently leads to cervical abnormalities and cancer. Moreover, HIV infection significantly decreases cervical cancer survival among HIV-positive women in Botswana [40].
3. Socio-economic status
Low socioeconomic status (SES) has been associated with an increased risk of cervical cancer in studies from both developing and developed countries [41–43]. A case-control study in the United States reported a 1.8-fold increased risk of cervical cancer among women who reside in poor counties in Ohio compared to those who reside in affluent counties [42]. El-moselhy et al. reported that women with low education level, unskilled, and reside in rural areas in Egypt were more likely to develop cervical cancer than those with high SES [43]. In Tanzania, women with no formal education were 4.30, (95% CI 3.50–5.31) increased the risk of cervical cancer compared to women with high education level [44]. Moreover, women with low SES were 2.3-fold more likely to die from cervical cancer compared to those with high SES [42]. Differences in SES as defined by education, occupation, and annual income play a major role in disparities in the incidence and mortality of cervical cancer. Women with low SES tend to engage in risky sexual behaviour like prostitution [45], which increases the risk of contracting sexually transmitted diseases (STDs) like HPV and HIV, which are important risk factors of cervical cancer. Moreover, women with low SES tend to lack health-seeking behavior [46], hence they are less likely to participate in cancer screening programmes.
4. Age at first sexual intercourse
Early age at first sexual intercourse is associated with risky sexual behaviour like having unprotected sex and multiple sexual partners, which are important risk factors of HPV and HIV [47]. A number of studies have reported an increased risk of cervical cancer with an early age at first sexual intercourse [48–50]. In Nigeria, women with an early onset of sexual intercourse ≤17 years were 3.7-fold increased risk of cervical cancer compared to those aged ≥ 18 years old [49]. A case-control study in Ethiopia indicated that women who experience sexual intercourse earlier than 15 years were 6.7-times more likely to develop cervical cancer than women who experience sexual intercourse between the ages of 21–25 years [50]. Compared with women with age at first sexual intercourse of ≥18 years, the odds ratio of developing cervical cancer was 1.90 and 2.60 among women with age at first sexual intercourse between the ages of 16–17 and <15 years old, respectively [51]. Previous studies have suggested that immature cervix is susceptible to high-risk HPV subtypes, which lead to persistent HPV infection and subsequently increased the risk of cervical cancer among young women [52].
5. Multiple sexual partners
Sexual activities are the main risk factors of HPV infection especially among those with multiple sexual partners [32,50]. Kassa et al. reported a 5.86-fold increased risk of cervical cancer among women with multiple sexual partners in Ethiopia [50]. In Eswatini, Jolly et al. reported an odds ratio 3.00, (95% CI 1.02–8.85) of developing cervical cancer among women with multiple sexual partners after adjusting for age at first sexual intercourse [32]. Moreover, a meta-analysis study suggested that having multiple sexual partners, with or without HPV infection, is an important risk factor of cervical cancer among sexually active women [53].
6. Multi-parity and early pregnancy
Multi-parity has been associated with an increased risk of cervical cancer [46,54]. Women who engage in early sexual intercourse may become pregnant at a young age and are more likely to be parous later in life [55]. Early pregnancies have been associated with an increased risk of cervical cancer [49]. The increased risk of cervical cancer among women with early pregnancies may be due to cervical trauma experienced during early childbearing or by high parity births [55]. A pooled analysis of eight case-control studies indicated that women who were parous at a young age were more likely to develop cervical cancer later in life [56]. Louie et al. suggested that the increased risk of cervical cancer among highly parous women may be attributed to sexual and reproductive events occurring at a young age [55].
7. Oral contraceptive use
Long-term use of oral contraceptives has been associated with cervical cancer risk [50,57]. In Ethiopia and Kenya, women with long-term use of oral contraceptives were associated with an increased risk of cervical cancer compared to women who do not use oral contraceptives [50,57]. A meta-analysis study of 28 case-control studies concluded that long-term use of oral contraceptive is an important risk factor of cervical cancer [58]. Moreover, an animal model study indicated that mouse treated with longer duration of estrogen developed cervical cancer than mouse treated with short duration of estrogen [59]. Interestingly, the incidence of cervical cancer has been found to decline over time as women stopped using oral contraceptives [60,61]. These findings suggest that long-term use of oral contraceptives is an important risk factor of cervical cancer especially among women of reproductive age.
8. Cigarette smoking
While cigarette smoking is commonly associated with lung cancer, it also plays a crucial role in carcinogenesis of many other cancers, including cervical cancer [62]. The International Agency for Research on Cancer classified tobacco as a group one carcinogen. A pooled analysis study revealed an increased risk of cervical cancer in current smokers as compared to non-smokers after adjusting for HPV infection and other environmental factors [63]. Min et al. showed that the risk of cervical cancer increased not only in women who smoke but also in women who are exposed to second-hand smoke [64]. The biological mechanism that underlies the increased risk of cervical cancer among women who smoke is not completely understood. However, studies have suggested that smoking inhibits the clearance of HPV infection by the immune system [65,66]. Surprisingly, the association between cigarette smoking and cervical cancer is stronger in SCC than in ADC [67]. The increased risk of SCC among women who smoke is not fully understood as it has rarely been investigated. However, quitting cigarette smoking has been reported to be associated with a decreased risk of cervical cancer [68]. Roura et al. reported a 2-fold decreased risk of developing cervical cancer among women who quit cigarette smoking.
9. Host genetic factors
Approximately 1% of women with chronic HPV infection progress to cervical cancer [69]. Magnuson et al. estimated that 27% of all cervical cancers are attributed to host genetic factors [70]. Studies have identified a number of single nucleotide polymorphisms (SNPs) mainly within the immune response genes to be associated with cervical cancer [71–74]. In Tunisia, a case-control study identified three SNPs (rs1800871, rs1800872, and rs3024490) within IL10 to be associated with an increased risk of cervical cancer [71]. In South Africa, CCR2-V64L G>A was associated with an increased risk of cervical cancer [72]. Zida et al. and Ben et al. identified TNF-α-308G>A and HLA-DRB1*15 and DQB1*06 to be associated with cervical carcinogenesis among HPV-negative women in Tunisia [73,74]. Apart from the immune response genes been heavily associated with cervical cancer, TP53 has also been implicated in cervical tumorigenesis. A candidate gene study in the black South African population indicated that TP53 rs1042522 SNP is associated with cervical cancer in HPV-negative women [75]. This finding reveals the direct role of host genetic factors in the aetiology of cervical cancer. However, the association between host genetic factors and cervical cancer has rarely been investigated in sub-Saharan Africa. Hence, more genetic studies with larger sample sizes and probably using genome-wide approaches are needed in sub-Saharan Africa to fully understand the aetiology of cervical cancer.
10. Cervical microbiome
Dysbiosis of the cervical microbiome has been associated with cervical cancer risk [76–78]. In Tanzania, Klein et al. reported that Staphylococcus, Pseudomonadales and Mycoplasmatales species were associated with high-grade squamous intraepithelial lesions in HIV-positive women [76]. Curty et al. found Gardnerella, Aerococcus, Schlegelella, Moryella, and Bifidobacterium to be associated with cervical lesions [77]. Moreover, a case-control study by Oh et al.
reported that Atopobium vaginae, Gardnerella vaginalis, and Lactobacillus iners were associated with an increased risk of cervical cancer [78]. Vagina and ectocervix are normally colonized by Lactobacillus species, which inhibit the growth of other bacterial species [79]. Inhibition of these bacteria species is crucial in maintaining the cervical epithelial barrier to HPV entry. However, reduction in the number of Lactobacillus species, result in colonization of cervical epithelium by other bacteria species [80]. These bacterial species produce enzymes and metabolites that compromise the cervical epithelium barrier [81], thus facilitating HPV entry into basal keratinocytes. In Kenya, bacterial vaginosis, trichomonas vaginosis, and Candida species were associated with high-risk HPV infection [82], which is an important risk factor of cervical cancer. Moreover, fungal and some of the bacterial species have been reported to enable HPV persistent, thus leading to cervical cancer [83].
Prevention and screening
1. HPV vaccination
HPV vaccination is becoming the primary prevention of cervical cancer and other HPV-associated cancers. Currently, the Food and Drug Administration approved three HPV vaccines including Gardasil 9, Gardasil, and Cervarix [84]. Gardasil 9 is a nanovalent vaccine produced by Merck [85]. This vaccine target nine HPV subtypes, seven of which are high-risk (16, 18, 31, 33, 45, 52 and 58) and two of which are low-risk (6 and 11). This vaccine target HPV subtypes that cause ≥ 90% of HPV-associated cancers worldwide [86]. Gardasil (Merck and Kenilworth) is a quadrivalent vaccine, thus targeting two high-risk subtypes (16 and 18) and two low-risk subtypes (6 and 11), and covering ≥ 80% of HPV-associated cancers [87]. Cervarix is a bivalent HPV vaccine produced by GlaxoSmithKline, targeting two high-risk HPV subtypes 16 and 18, which contribute 70% of cervical cancer worldwide [87].
However, these vaccines cannot prevent pre-existing infections and are administered to pre-adolescent girls between the ages of 9–15 years [84]. Uptake of these vaccines is low in developing countries, especially in sub-Saharan Africa [88]. However, most countries are now implementing HPV vaccination in school-going children. It is estimated that ≥ 6.7 million cervical cancer cases could be prevented if HPV vaccination coverage is scaled up to 80–100% globally by the year 2020 [9]. However, it will take several decades in sub-Saharan Africa to see the impact of HPV vaccination.
2. Pap smear test
Pap smear is a screening test used to check cervical lesions. It is usually performed in women of reproductive age and who are sexually active [89]. Pap smear can detect early cervical dysplasia in its earliest form. Once cervical abnormalities are detected, the best way is to treat the precancerous lesions before they can fully develop into cancer. Countries that have successfully implemented cervical cancer screening have also significantly reduced both the incidence and mortality of cervical cancer, especially SCC [90,91]. The HPV DNA test has also been recommended to be included in cervical cancer screening package among women of reproductive age [89]. Cervical cytology together with HPV DNA test has higher sensitivity than screening with Pap smear alone [92]. A recent study suggested that rapid scale-up of HPV vaccination and screening from 2020 onwards will rapidly decrease the incidence and mortality of cervical cancer in the next five decades [9]. However, cervical cancer screening coverage is still low in sub-Saharan Africa [93], and there is a need for rapid scale-up to avert the high incidence and mortality of cervical cancer.
3. Visual inspection with acetic acid (VIA)
VIA is an alternative cervical cancer screening method viable in sub-Saharan Africa, as it is cheap, easy to use, does not require a physician or pathologist to perform, and tend to have shorter turn-around time than Pap smear [94]. In VIA, about 5% acetic acid (vinegar) is applied to the cervix and then visualized with a lamp. The precancerous lesions on the cervix normally turn white when combined with acetic acid whilst normal cervix do not change colour [95]. A number of studies in sub-Saharan Africa have indicated that VIA has a higher sensitivity than Pap smear [96–99]. Specifically, VIA has a high sensitivity for SCC which contribute ≥ 80% of cervical cancers in sub-Saharan Africa [97]. However, previous studies have indicated that VIA tends to have lower specificity than Pap smear [96–98]. Nonetheless, VIA is comparable to Pap smear and the World Health Organization recommended VIA as a primary cervical cancer screening method in developing countries, where pathology laboratories are limited. The low cervical cancer screening rate in sub-Saharan Africa may be attributed to inadequate funding, lack of awareness campaign, lack of health-seeking behaviour, low SES, and long-distance to access healthcare facility [100]. Cervical cancer can also be prevented by having safe sex, monogamous relationship and adopting a healthy lifestyle like quitting cigarette smoking, alcohol consumption and engaging in regular physical activity.
Summary and conclusion
The incidence of cervical cancer is highest in sub-Saharan Africa. Epidemiological studies have indicated that infection with high-risk HPV subtypes is crucial in the carcinogenesis of cervical cancer. Apart from HPV infection, HIV, SES, age at first sexual intercourse, multiple sexual partners, oral contraceptive use, multi-parity, early pregnancies, cigarette smoking, host genetic factors, and dysbiosis of the cervical microbiome are also important risk factors associated with cervical cancer in sub-Saharan Africa. However, cervical cancer can be prevented by HPV vaccination and early detection through screening. Gardasil and Cervarix are two HPV vaccines that are currently used to prevent cervical cancer and other HPV-associated cancers in sub-Saharan Africa. Scaling up HPV vaccination and decentralization of cervical cancer screening programmes from tertiary-level to primary-level care is crucial in preventing the incidence and mortality of cervical cancer in sub-Saharan Africa. Moreover, future cervical cancer prevention programmes should include other risk factors associated with the disease.
Funding: None.
Conflicts of Interest: None
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68: 394–424. [Crossref]
- Chaturvedi AK, Kleinerman RA, Hildesheim A, Gilbert ES, Storm H, et al. (2009) Second Cancers After Squamous Cell Carcinoma and Adenocarcinoma of the Cervix. J Clin Oncol 27: 967–973.
- Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, et al. (2018) Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29. [Crossref]
- Silverberg SGM, Loffe OBM (2003) Pathology of Cervical Cancer. Cancer J 9: 335–347. [Crossref]
- Wang SS, Sherman ME, Hildesheim A, Lacey JV, Devesa S (2004) Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976–2000. Cancer 100: 1035–1044. [Crossref]
- Yokoi E, Mabuchi S, Takahashi R, Matsumoto Y, Kuroda H, et al. (2017) Impact of histological subtype on survival in patients with locally advanced cervical cancer that were treated with definitive radiotherapy: adenocarcinoma/adenosquamous carcinoma versus squamous cell carcinoma. J Gynecol Oncol 28. [Crossref]
- Baldur-Felskov B, Munk C, Nielsen TSS, Dehlendorff C, Kirschner B, et al. (2015) Trends in the incidence of cervical cancer and severe precancerous lesions in Denmark, 1997–2012. Cancer Causes Control 26: 1105–1116. [Crossref]
- Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. (2017) Global Cancer in Women: Burden and Trends. Cancer Epidemiol Biomarkers Prev 26: 444–457. [Crossref]
- Simms KT, Steinberg J, Caruana M, Smith MA, Lew J-B, et al. (2019) Impact of scaled up human papillomavirus vaccination and cervical screening and the potential for global elimination of cervical cancer in 181 countries, 2020–99: a modelling study. Lancet Oncol 20: 394–407.
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, et al. (1999) Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 189: 12–19. [Crossref]
- De Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, et al. (2010) Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 11: 1048–1056. [Crossref]
- Tjalma WA. (2006) Cervical cancer and prevention by vaccination: results from recent trials. Ann Oncol 17: 217–223. [Crossref]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348: 518–527. [Crossref]
- Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, et al. (2007) Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 121: 621–632. [Crossref]
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD (1998) Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 338: 423–428. [Crossref]
- McBride AA (2017) Oncogenic human papillomaviruses. Philos Trans R Soc Lond B Biol Sci 372.
- Chen J (2015) Signaling pathways in HPV-associated cancers and therapeutic implications: Signaling pathways in HPV-associated cancers. Reviews in Medical Virology 25: 24–53. [Crossref]
- Yugawa T, Kiyono T (2009) Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Reviews in Medical Virology 19: 97–113. [Crossref]
- Senba M, Mori N (2012) Mechanisms of virus immune evasion lead to development from chronic inflammation to cancer formation associated with human papillomavirus infection. Oncol Rev 6. [Crossref]
- Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Future Virol 6: 45–57.
- Tomaić V (2016) Functional Roles of E6 and E7 Oncoproteins in HPV-Induced Malignancies at Diverse Anatomical Sites. Cancers (Basel) 8. [Crossref]
- Gul S, Murad S, Javed A (2015) Prevalence of High risk Human Papillomavirus in cervical dysplasia and cancer samples from twin cities in Pakistan. International Journal of Infectious Diseases 34: 14–19. [Crossref]
- Burd EM (2003) Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev 16: 1–17.
- Bonello K, Blundell R (2016) The Role of the Human Papillomavirus (HPV) in Cervical Cancer: A Review about HPV-Induced Carcinogenesis and Its Epidemiology, Diagnosis, Management and Prevention. International Journal of Medical Students 4: 26–32.
- Hoory T, Monie A, Gravitt P, Wu TC (2008) Molecular Epidemiology of Human Papillomavirus. Journal of the Formosan Medical Association 107: 198–217. [Crossref]
- Furumoto H, Irahara M (2002) Human papilloma virus (HPV) and cervical cancer. J Med Invest 49: 124–133.
- Ghittoni R, Accardi R, Chiocca S, Tommasino M (2015) Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience 9: 526. [Crossref]
- Van der Marel J, Berkhof J, Ordi J, Torné A, Del Pino M, et al. (2015) Attributing oncogenic human papillomavirus genotypes to high-grade cervical neoplasia: which type causes the lesion? Am J Surg Pathol 39: 496–504. [Crossref]
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, et al. (2006) Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 20: 1645–1654. [Crossref]
- Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA (2009) Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 101: 1120–1130. [Crossref]
- Whitham HK, Hawes SE, Chu H, Oakes JM, Lifson AR, et al. (2017) A Comparison of the Natural History of HPV Infection and Cervical Abnormalities among HIV-Positive and HIV-Negative Women in Senegal, Africa. Cancer Epidemiol Biomarkers Prev 26: 886–894. [Crossref]
- Jolly PE, Mthethwa-Hleta S, Padilla LA, Pettis J, Winston S, et al. (2017) Screening, prevalence, and risk factors for cervical lesions among HIV positive and HIV negative women in Swaziland. BMC Public Health 17: 218. [Crossref]
- Singh DK, Anastos K, Hoover DR, Burk RD, Shi Q, et al. (2009) Human papillomavirus infection and cervical cytology in HIV-infected and HIV-uninfected Rwandan women. J Infect Dis 199: 1851–1861. [Crossref]
- Ng’andwe C, Lowe JJ, Richards PJ, Hause L, Wood C, et al. (2007) The distribution of sexually-transmitted Human Papillomaviruses in HIV positive and negative patients in Zambia, Africa. BMC Infect Dis 7: 77. [Crossref]
- Chirenje ZM, Loeb L, Mwale M, Nyamapfeni P, Kamba M, et al. (2002) Association of cervical SIL and HIV-1 infection among Zimbabwean women in an HIV/STI prevention study. Int J STD AIDS 13: 765–768.
- Moodley JR, Constant D, Hoffman M, Salimo A, Allan B, et al. (2009) Human papillomavirus prevalence, viral load and pre-cancerous lesions of the cervix in women initiating highly active antiretroviral therapy in South Africa: a cross-sectional study. BMC Cancer 9: 275. [Crossref]
- Looker KJ, Rönn MM, Brock PM, Brisson M, Drolet M, et al. (2018) Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc 21. [Crossref]
- Williamson AL (2015) The Interaction between Human Immunodeficiency Virus and Human Papillomaviruses in Heterosexuals in Africa. J Clin Med 4: 579–592. [Crossref]
- Kelly HA, Sawadogo B, Chikandiwa A, Segondy M, Gilham C, et al. (2017) Epidemiology of high-risk human papillomavirus and cervical lesions in African women living with HIV/AIDS: effect of anti-retroviral therapy. AIDS 31: 273–285. [Crossref]
- Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, Efstathiou JA, Grover S, et al. (2016) HIV Infection and Survival Among Women With Cervical Cancer. J Clin Oncol 34: 3749–3757.
- Irimie S, Vlad M, Mirestean IM, Balacescu O, Rus M, et al. (2011) Risk Factors in a Sample of Patients with Advanced Cervical Cancer. Applied Medical Informatics 29: 1–10.
- Kollman J (2018) Poverty and Cancer Disparities in Ohio. Prev Chronic Dis 15.
- Ea E-M, Hm B, Sa A (2016) Cervical Cancer: Sociodemographic and Clinical Risk Factors among Adult Egyptian Females. Journal of Oncology Research and Treatment 1: 1–7.
- Kahesa C, Kjaer SK, Ngoma T, Mwaiselage J, Dartell M, et al. (2012) Risk factors for VIA positivity and determinants of screening attendances in Dar es Salaam, Tanzania. BMC Public Health 12: 1055. [Crossref]
- DINKELMAN T, LAM D, LEIBBRANDT M (2008) LINKING POVERTY AND INCOME SHOCKS TO RISKY SEXUAL BEHAVIOUR. S Afr J Econ 76: 52–74.
- Mebratie AD, Poel EV de, Yilma Z, Abebaw D, Alemu G, et al. (2014) Healthcare-seeking behaviour in rural Ethiopia: evidence from clinical vignettes. BMJ Open 4.
- Zhang R, Shi TY, Ren Y, Lu H, Wei Z-H, et al. (2013) Risk factors for human papillomavirus infection in Shanghai suburbs: a population-based study with 10,000 women. J Clin Virol 58: 144–148. [Crossref]
- Adewuyi SA, Shittu SO, Rafindadi AH (2008) Sociodemographic and clinicopathologic characterization of cervical cancers in northern Nigeria. Eur J Gynaecol Oncol 29: 61–64. [Crossref]
- Ogunbowale T, Lawoyin TO (2008) Cervical cancer risk factors and predictors of cervical dysplasia among women in south-west Nigeria. Aust J Rural Health 16: 338–342. [Crossref]
- Kassa RT (2018) Risk factors associated with precancerous cervical lesion among women screened at Marie Stops Ethiopia, Adama town, Ethiopia 2017: a case control study. BMC Res Notes 11: 145. [Crossref]
- Williams MA, Kenya PR, Mati JK, Thomas DB (1994) Risk factors for invasive cervical cancer in Kenyan women. Int J Epidemiol 23: 906–912. [Crossref]
- Moscicki AB, Winkler B, Irwin CE, Schachter J (1989) Differences in biologic maturation, sexual behavior, and sexually transmitted disease between adolescents with and without cervical intraepithelial neoplasia. The Journal of Pediatrics 115: 487–493. [Crossref]
- Liu ZC, Liu WD, Liu YH, Ye XH, Chen SD (2015) Multiple Sexual Partners as a Potential Independent Risk Factor for Cervical Cancer: a Meta-analysis of Epidemiological Studies. Asian Pac J Cancer Prev 16: 3893–3900. [Crossref]
- Bezabih M, Tessema F, Sengi H, Deribew A (2015) Risk Factors Associated with Invasive Cervical Carcinoma among Women Attending Jimma University Specialized Hospital, Southwest Ethiopia: A Case Control Study. Ethiop J Health Sci 25: 345–352.
- Louie KS, de Sanjose S, Diaz M, Castellsagué X, Herrero R, et al. (2009) Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer 100: 1191–1197. [Crossref]
- Muñoz N, Franceschi S, Bosetti C, Moreno V, Herrero R, et al. (2002) Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. The Lancet 359: 1093–1101. [Crossref]
- Soh J, Rositch AF, Koutsky L, Guthrie BL, Choi RY, et al. (2014) Individual and partner risk factors associated with abnormal cervical cytology among women in HIV-discordant relationships. Int J STD AIDS 25: 315–324. [Crossref]
- Smith JS, Green J, Berrington de Gonzalez A, Appleby P, Peto J, et al. (2003) Cervical cancer and use of hormonal contraceptives: a systematic review. Lancet 361: 1159–1167. [Crossref]
- Brake T, Lambert PF (2005) Estrogen contributes to the onset, persistence, and malignant progression of cervical cancer in a human papillomavirus-transgenic mouse model. Proc Natl Acad Sci USA 102: 2490–2495. [Crossref]
- Bayo S, Bosch FX, de Sanjosé S, Muñoz N, Combita AL, et al. (2002) Risk factors of invasive cervical cancer in Mali. Int J Epidemiol 31: 202–209. [Crossref]
- Muwonge R, Ngo Mbus L, Ngoma T, Gombe Mbalawa C, Dolo A, et al. (2016) Socio-demographic and reproductive determinants of cervical neoplasia in seven sub-Sahara African countries. Cancer Causes Control 27: 1437–1446. [Crossref]
- Fonseca-Moutinho JA (2011) Smoking and Cervical Cancer. ISRN Obstet Gynecol 2011.
- Plummer M, Herrero R, Franceschi S, Meijer CJLM, Snijders P, et al. (2003) Smoking and cervical cancer: pooled analysis of the IARC multi-centric case–control study. Cancer Causes Control 14: 805–814. [Crossref]
- Min KJ, Lee JK, So KA, Kim MK (2018) Association Between Passive Smoking and the Risk of Cervical Intraepithelial Neoplasia 1 in Korean Women. J Epidemiol 28: 48–53. [Crossref]
- Giuliano AR, Sedjo RL, Roe DJ, Harri R, Baldwi S, et al. (2002) Clearance of oncogenic human papillomavirus (HPV) infection: effect of smoking (United States). Cancer Causes Control 13: 839–846. [Crossref]
- Koshiol J, Schroeder J, Jamieson DJ, Marshall SW, Duerr A, et al. (2006) Smoking and time to clearance of human papillomavirus infection in HIV-seropositive and HIV-seronegative women. Am J Epidemiol 164: 176–183. [Crossref]
- Berrington de González A, Sweetland S, Green J (2004) Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis. Br J Cancer 90: 1787–1791. [Crossref]
- Roura E, Castellsagué X, Pawlita M, Travier N, Waterboer T, et al. (2014) Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. International Journal of Cancer 135: 453–466. [Crossref]
- Xu H-H, Zhang X, Zheng H-H, Han Q-Y, Lin A-F, et al. (2018) Association of HLA-G 3′ UTR polymorphism and expression with the progression of cervical lesions in human papillomavirus 18 infections. Infect Agent Cancer 13: 42. [Crossref]
- Magnusson PKE, Lichtenstein P, Gyllensten UB (2000) Heritability of cervical tumours. International Journal of Cancer 88: 698–701. [Crossref]
- Zidi S, Gazouani E, Stayoussef M, Mezlini A, Ahmed SK, et al. (2015) IL-10 gene promoter and intron polymorphisms as genetic biomarkers of cervical cancer susceptibility among Tunisians. Cytokine 76: 343–347. [Crossref]
- Chatterjee K, Dandara C, Hoffman M, Williamson AL (2010) CCR2-V64I polymorphism is associated with increased risk of cervical cancer but not with HPV infection or pre-cancerous lesions in African women. BMC Cancer 10: 278. [Crossref]
- Zidi S, Stayoussef M, Zouidi F, Benali S, Gazouani E, et al. (2015) Tumor Necrosis Factor Alpha (-238 / -308) and TNFRII-VNTR (-322) Polymorphisms as Genetic Biomarkers of Susceptibility to Develop Cervical Cancer Among Tunisians. Pathol Oncol Res 21: 339–345.
- Ben Othmane Y, Ghazouani E, Mezlini A, Lagha A, Raïs M, et al. (2012) HLA class II susceptibility to cervical cancer among Tunisian women. Bull Cancer 99: 81–86. [Crossref]
- Pegoraro RJ, Rom L, Lanning PA, Moodley M, Naiker S, et al. (2002) P53 codon 72 polymorphism and human papillomavirus type in relation to cervical cancer in South African women. Int J Gynecol Cancer 12: 383–388. [Crossref]
- Klein C, Gonzalez D, Samwel K, Kahesa C, Mwaiselage J, et al. (2019) Relationship between the Cervical Microbiome, HIV Status, and Precancerous Lesions. MBio 10. [Crossref]
- Curty G, Costa RL, Siqueira JD, Meyrelles AI, Machado ES, et al. (2017) Analysis of the cervical microbiome and potential biomarkers from postpartum HIV-positive women displaying cervical intraepithelial lesions. Sci Rep 7.
- Oh HY, Kim BS, Seo SS, Kong JS, Lee JK, et al. (2015) The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect 21: 674. [Crossref]
- Witkin SS, Linhares IM (2017) Why do lactobacilli dominate the human vaginal microbiota? BJOG: An International Journal of Obstetrics & Gynaecology 124: 606–611. [Crossref]
- Amabebe E, Anumba DOC (2018) The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front Med (Lausanne) 5: 181. [Crossref]
- Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, et al. (2016) The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 4: 58.
- Menon S, Broeck DV, Rossi R, Ogbe E, Harmon S, et al. (2016) Associations Between Vaginal Infections and Potential High-risk and High-risk Human Papillomavirus Genotypes in Female Sex Workers in Western Kenya. Clin Ther 38: 2567–2577. [Crossref]
- Uren A, Fallen S, Yuan H, Usubütün A, Küçükali T, et al. (2005) Activation of the canonical Wnt pathway during genital keratinocyte transformation: a model for cervical cancer progression. Cancer Res 65: 6199–6206. [Crossref]
- Harper DM, DeMars LR (2017) HPV vaccines – A review of the first decade. Gynecologic Oncology 146: 196–204. [Crossref]
- Zhang Z, Zhang J, Xia N, Zhao Q (2017) Expanded strain coverage for a highly successful public health tool: Prophylactic 9-valent human papillomavirus vaccine. Hum Vaccin Immunother 13: 2280–2291.
- Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, et al. (2012) Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agents Cancer 7: 38. [Crossref]
- Gattoc L, Nair N, Ault K (2013) Human Papillomavirus Vaccination. Obstet Gynecol Clin North Am 40: 177–197.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, et al. (2016) Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 4: 453–463. [Crossref]
- Jeronimo J, Castle PE, Temin S, Denny L, Gupta V, et al. (2016) Secondary Prevention of Cervical Cancer: ASCO Resource-Stratified Clinical Practice Guideline. JGO 3: 635–657. [Crossref]
- Chiang YC, Chen YY, Hsieh SF, Chiang CJ, You SL, et al. (2017) Screening frequency and histologic type influence the efficacy of cervical cancer screening: A nationwide cohort study. Taiwanese Journal of Obstetrics and Gynecology 56: 442–448. [Crossref]
- Murillo R, Herrero R, Sierra MS, Forman D (2016) Cervical cancer in Central and South America: Burden of disease and status of disease control. Cancer Epidemiology 44: 121–130. [Crossref]
- Moy LM, Zhao FH, Li LY, Ma JF, Zhang QM, et al. (2010) Human papillomavirus testing and cervical cytology in primary screening for cervical cancer among women in rural China: Comparison of sensitivity, specificity, and frequency of referral. International Journal of Cancer 127: 646–656. [Crossref]
- Gakidou E, Nordhagen S, Obermeyer Z (2008) Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med 5: 132. [Crossref]
- Saleh HS (2014) Can visual inspection with acetic acid be used as an alternative to Pap smear in screening cervical cancer? Middle East Fertility Society Journal 19: 187–191.
- Kavita SN, Shefali M (2010) Visual inspection of cervix with acetic acid (VIA) in early diagnosis of cervical intraepithelial neoplasia (CIN) and early cancer cervix. J Obstet Gynaecol India 60: 55–60.
- Doh AS, Nkele NN, Achu P, Essimbi F, Essame O, et al. (2005) Visual inspection with acetic acid and cytology as screening methods for cervical lesions in Cameroon. International Journal of Gynecology & Obstetrics 89: 167–173. [Crossref]
- Goel A, Gandhi G, Batra S, Bhambhani S, Zutshi V, et al. (2005) Visual inspection of the cervix with acetic acid for cervical intraepithelial lesions. Int J Gynaecol Obstet 88: 25–30. [Crossref]
- Ardahan M, Temel AB (2011) Visual inspection with acetic acid in cervical cancer screening. Cancer Nurs 34: 158–163.
- Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, et al. (2008) Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer 123: 153–160. [Crossref]
- Birhanu Z, Abdissa A, Belachew T, Deribew A, Segni H, et al. (2012) Health seeking behavior for cervical cancer in Ethiopia: a qualitative study. International Journal for Equity in Health 11: 83. [Crossref]